Differential distribution of vesicle associated membrane protein isoforms in the mouse retina
|
|
|
- Michael Fowler
- 6 years ago
- Views:
Transcription
1 Received 21 August 2003 Accepted 24 November 2003 Published 11 December 2003 Differential distribution of vesicle associated membrane protein isoforms in the mouse retina David M. Sherry, Meng M. Wang, Laura J. Frishman College of Optometry, University of Houston, Houston, TX Purpose: Many proteins associated with synaptic vesicle exocytosis are differentially distributed among synapses in the retina and elsewhere in the central nervous system. The synapse-specific distribution of these proteins and their isoforms is thought to contribute to synapse-specific functional differences. Vesicle-associated membrane protein (VAMP, also known as synaptobrevin) is an integral synaptic vesicle membrane protein that is part of the fusion core complex needed for docking and fusing of synaptic vesicles at the synaptic active zone. Two VAMP isoforms have been identified that are considered to be synaptic, VAMP-1 and VAMP-2, however their distributions among the various synapses in the mammalian retina have not been characterized. Methods: Single- and double-labeling immunocytochemistry was used to investigate the distribution of the synaptic VAMP isoforms, VAMP-1 and VAMP-2, in the mouse retina. Results: VAMP-2 was the predominant isoform in both synaptic layers. Double-labeling studies using conventional and ribbon-synapse-specific markers showed that VAMP-2 was broadly distributed among conventional and ribbon synapses. In contrast, the distribution of VAMP-1 was very limited. In the outer retina, only weak labeling was present in photoreceptor terminals. In the inner retina, labeling for VAMP-1 was found in the dendrites, cell bodies, and axons of some ganglion cells, as demonstrated by double labeling with the ganglion cell markers, microtubule-associated protein-1 and Brn-3a. VAMP-1 labeling did not colocalize with amacrine or bipolar cell markers, nor did it colocalize with other presynaptic markers, suggesting that VAMP-1 is not associated directly with neurotransmitter release in the inner retina. Labeling for VAMP-1 identified a set of large ganglion cells that ramified in the mid-ipl (inner plexiform layer), suggesting that they may show ON-OFF responses. Some of these cells had cell bodies displaced to the inner nuclear layer. The dendrites of the large VAMP-1-immunoreactive ganglion cells did not o-stratify with the cholinergic plexuses of the starburst amacrine cells (labeled for choline acetyltransferase) and therefore are unlikely to show directional selectivity. However, these cells are likely to receive input from bipolar cells and a population of putative glutamatergic amacrine cells. Conclusions: VAMP-1 and VAMP-2 are differentially distributed among the synapses of the mouse retina. VAMP-2 is the predominant isoform and is widely expressed at ribbon and conventional synapses in both plexiform layers. VAMP-1 expression in the mouse retina is much more limited and is not restricted to presynaptic terminals. In the OPL, VAMP-1 is co-expressed with VAMP-2 presynaptically in photoreceptor terminals. However, VAMP-1 expression in the IPL is associated with ganglion cells and does not appear to be localized to presynaptic terminals. VAMP-1 is a specific marker for a set of large ganglion cells and displaced ganglion cells that ramify in the mid-ipl and are likely to have ON-OFF physiology. The presynaptic proteins regulating synaptic vesicle exocytosis and neurotransmitter release have been the subject of intense study in recent years. Many of these proteins exist in multiple isoforms and are differentially distributed in a synapse-specific manner [1-3]. A particularly important protein associated with transmitter release at vesicular synapses is vesicle associated membrane protein (VAMP, also known as synaptobrevin). VAMP is an integral membrane protein located in the synaptic vesicle membrane and complexes with Correspondence to: David M. Sherry, University of Houston, College of Optometry, 505 J. Davis Armistead Building, Houston, TX, ; Phone: (713) ; FAX: (713) ; dsherry@uh.edu Meng M. Wang is currently at the University of California-Berkeley, Department of Molecular and Cell Biology, 335 LSA 3200, Berkeley, CA, two other proteins associated with the presynaptic plasma membrane, syntaxin and SNAP-25, to form the core complex that docks synaptic vesicles at the active zone of the synapse and provides the protein scaffold needed for subsequent membrane fusion [1,2]. Two VAMP isoforms, VAMP-1 and VAMP-2, are considered to be synapse-specific in the central nervous system [4-6]. Multiple VAMP isoforms are likely to be expressed in the mammalian retina. Previous studies indicate that VAMP-2 is present in the mammalian retina [7,8], however, the distribution of other VAMP isoforms has not been examined. At least two VAMP isoforms are differentially localized in the salamander retina [9]. Although data regarding any VAMP isoforms other than VAMP-2 in the mammalian retina are currently lacking, several proteins with which VAMP interacts are known to have more than one isoform present in the retina. Two isoforms of
2 TABLE 1. PRIMARY ANTIBODIES AND ANTISERA Antigen Host Dilution Source VAMP-1 Rabbit 1:500-1:1000 Synaptic Systems, Göttingen, Germany VAMP-2 Rabbit 1:1000-1:50,000 Synaptic Systems, Göttingen, Germany VAMP-2 Mouse 1:500-1:1000 Synaptic Systems, Göttingen, Germany [14] Vesicular Rabbit 1:1000 Synaptic Glutamate Systems, Transporter 1 Göttingen, (VGLUT1) Germany VGLUT1 Guinea 1:5000 Chemicon Pig International, Temecula, CA VGLUT3 Guinea 1:2500 Chemicon Pig International, Temecula, CA Calbindin (CaBP) Mouse 1:300 Sigma Chemical Company, St. Louis, MO Synapsin I Mouse 1:100 Chemicon International, Temecula, CA [63] syntaxin, another core complex protein, have been localized in the rat retina [7]. Syntaxin 1 is present at conventional synapses that typically release gamma-aminobutyric acid (GABA) or glycine transiently; syntaxin 3 is expressed at ribbon synapses, which release glutamate at high rates. The other core complex protein, SNAP-25, also may exist in more than one isoform in the mammalian retina, although this result is somewhat controversial. SNAP-25 localization in the mammalian retina has varied markedly among investigators using different SNAP-25 antibodies [8,10-13], suggesting multiple SNAP- 25 isoforms may be present. However, unlike syntaxin isoforms, there are no clear indications of different SNAP-25 isoforms in conventional vs. ribbon synapses. Synaptophysin, which interacts with VAMP to regulate its availability for core complex formation [14], and its homologue, synaptoporin, also are differentially distributed among synapses in the rat and rabbit retina [15]. Other presynaptic proteins associated with transmitter exocytosis and synaptic vesicle trafficking also display isoformand synapse-specific distribution in the mammalian retina including: rabphilin and synapsins, which are restricted to conventional synapses [8,16], and isoforms of synaptic vesicle protein 2 (SV2; 8,17), amphiphysin [8], and synaptotagmin [17-19], which are all differentially distributed among ribbon and conventional synapses. Differential expression of these proteins and their isoforms are thought to confer synapse-specific differences in functional transmitter functional properties [1-3]. To better understand the potential role of VAMPs at retinal synapses, we have examined the distribution of VAMP isoforms in the mouse retina using immunocytochemical methods and antibodies specific for VAMP-1 and VAMP-2. These studies indicate that at least two synaptic VAMP isoforms are present in the mammalian retina and are differentially distributed, with VAMP-2 being distributed widely among both ribbon and conventional synapses, and VAMP-1 being restricted to photoreceptor ribbon synapses. In addition, these studies show that VAMP-1-immunoreactivity that is not directly associated with transmitter release, is an excellent marker for a subset of large ganglion cells. Protein Kinase Rabbit 1:50-1:100 Calbiochem, San Cα (PKC) Diego, CA Synaptic Vesicle, Mouse 1:20-1:50 Dr. K. Buckley, Protein 2 (SV2) Harvard University, Boston, MA [30] Microtubule Mouse 1:300 Sigma Chemical Associated Company, St. Protein-1 (MAP-1) Louis, MO [64] Brn-3a Mouse 1:25-1:50 Chemicon International, Temecula, CA [29] Glutamic Acid Mouse 1:1000 Chemicon Decarboxylase, 65 kda International, isoform (GAD-65) Temecula, CA [65] Choline Goat 1:75-1:100 Chemicon Acetyl-transferase International, (CHAT) Temecula, CA [66] Glycine Rat 1:800-1:1,000 Dr. D. V. Pow, University of Queensland, Australia, [67] Summary of specificity, source, and dilution of primary antibodies used in these studies. 674 Figure 1. Immunoblots of VAMP-1 and VAMP-2 in mouse retina and brain. Immunoblotting of mouse retinal and brain membrane homogenates using antibodies directed against VAMP-2 and VAMP- 1 isoforms. The mouse monoclonal and rabbit polyclonal antibodies against VAMP-2 strongly labeled a single band at the expected molecular mass in retina and brain membrane homogenates. The rabbit polyclonal VAMP-1 antiserum showed strong labeling of the expected band in brain membrane homogenates, but produced only faint labeling in retinal membrane homogenates, consistent with the limited distribution of VAMP-1 in the retina.
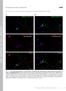
3 METHODS Animals and tissue preparation: Adult mice were euthanized by injection of a lethal dose of pentobarbital (100 mg/kg). All animal procedures conformed to US Public Health Service and Institute for Laboratory Animal Research guidelines and were approved by the University of Houston Institutional Animal Care and Use Committee. The eyes were rapidly excised from the head, leaving a portion of the superior rectus muscle attached as a landmark to indicate the superior pole of the globe, the corneas were slit with a razor blade, and the eyes were immediately immersed in 4% formaldehyde in 0.1 M cacodylate buffer (ph 7.4) overnight at 4 C. Following fixation, eyes were rinsed, embedded in OCT embedding medium, and fast frozen. Vertical cryostat sections of µm thickness were taken along the vertical meridian of the eyecup and collected onto gelatin-coated slides. Sections were stored at -20 C until use. All animals used for these studies were light adapted. Twenty two mice of three different strains were used, C57BL/6 (n =17); 129 (n=4); and CD-1 (n=1). Antibodies and antisera: Isoform-specific antibodies directed against VAMP-1 or VAMP-2 were used in these studies. To label VAMP-1, a rabbit polyclonal antiserum specific for the N-terminus of VAMP-1 (amino acids 2-14) was used. For most studies of VAMP-2, a mouse monoclonal antibody directed against the N-terminus region of the VAMP-2 molecule [14] was used. A rabbit polyclonal antibody specific for the N-terminus of VAMP-2 (amino acids 2-17) also was used for some studies and yielded identical results. The isoform specificity of these antibodies for rat brain VAMP-1 and VAMP-2, which have N-terminus amino acid sequences identical to those in mouse, has been described previously [9]. To Figure 2. VAMP-1 and VAMP-2 are differentially distributed in the mouse retina. A: Intense labeling for VAMP-2 is present in both the OPL and IPL. Photoreceptor terminals (small arrowheads) in the OPL show strong labeling. Numerous terminals throughout the IPL show intense labeling for VAMP-2, including a population of large terminals in the innermost portion of the IPL where the rod bipolar cell terminals reside (large arrowheads). A few slender processes that show VAMP-2 labeling (arrows), most likely bipolar cell axons, can be seen descending through the INL. B: Labeling for VAMP-1 is present in the OPL and IPL, but is more restricted in distribution than VAMP-2 labeling. Weak labeling of photoreceptor terminals (arrows) is visible in the OPL. Puncta labeled for VAMP-1 are distributed throughout the IPL (small arrowheads), but a broad plexus of prominently labeled puncta is present in the mid-ipl (square bracket symbol). An intensely labeled cell body (*) is visible in the ganglion cell layer (GCL). Axon bundles along the inner edge of the retina also show labeling (large arrowheads). Inset: VAMP-1 immunolabeling in the OPL. ONL, outer nuclear layer. Scale bars represent 20 µm for A and B; 10 µm for inset in B. 675
4 identify specific retinal synapses and cells containing VAMP- 1 and VAMP-2, antibodies directed against synapsin I, protein kinase Cα (PKC), synaptic vesicle protein 2 (SV2, panspecific), microtubule-associated protein 1 (MAP-1), Brn-3a, vesicular glutamate transporter 1 (VGLUT1), vesicular glutamate transporter 3 (VGLUT3), glycine, the 65 kda isoform of glutamic acid decarboxylase (GAD), and choline acetyltransferase (CHAT) were used. Table 1 provides the details of all primary antibodies used in these studies. Binding of primary antibodies was visualized using fluorescent secondary antisera. For most experiments, secondary antisera were raised in goat and were specific for mouse, rabbit, guinea pig, or rat immunoglobulins and were conjugated to either Cy3 (red fluorescence; diluted 1:500; Jackson ImmunoResearch Laboratories, West Grove, PA) or AlexaFluor 488 (green fluorescence; diluted 1:400-1:800; Molecular Probes, Eugene, OR). For double labeling, a combination of Cy3- and AlexaFluor 488-conjugated secondary antibodies was used. For double-labeling experiments using the goat anti-chat antiserum, secondary antisera raised in donkey and specific for goat or rabbit immunoglobulins conjugated to either Cy3 (diluted 1:500) or AlexaFluor 488 (diluted 1:500-1:800) were used. Immunoblotting: To assess the specificity of the VAMP antibodies, immunoblotting of retinal membranes was performed as previously described [9,19,20]. Briefly, proteins in mouse retina and brain membrane homogenates were resolved by SDS-PAGE using 12.5% polyacrylamide gel and then blotted onto PVDF membranes. Membranes were blocked, incubated with a VAMP primary antibody, rinsed and then incu- Figure 3. VAMP-2 is expressed at photoreceptor and bipolar cell ribbon synapses. A-C: Double-labeling for VAMP-2 and VGLUT1, a marker for rod and cone terminals in the OPL. Labeling for VAMP-2 colocalizes with labeling for VGLUT1, indicating that VAMP-2 is present in the terminals of rod and cone photoreceptors. D-F: Double-labeling for VAMP-2 and PKC, a marker for rod bipolar cell terminals in the IPL. Labeling for VAMP-2 and PKC colocalizes in the terminals of the rod bipolar cells (arrowheads). Rod bipolar cell axons (arrows) show little labeling for VAMP-2. G-I: Confocal microscopy of double-labeling for VAMP-2 and VGLUT1, a marker for all bipolar cell terminals. Terminals of cone bipolar cells from the mid-ipl are illustrated. Labeling for VAMP-2 colocalizes with VGLUT1, confirming that VAMP-2 is present in cone bipolar cell terminals (arrows) in addition to rod bipolar cells. Several conventional synaptic terminals showing only VAMP- 2 labeling (arrowheads) also are present. A single optical section of 0.29 µm thickness is shown. Scale bars represent 10 µm for A-F; 2 µm for G-I. 676
5 bated in secondary antibody. Immunolabeled protein bands were visualized on the blots using enhanced chemiluminescence and a Nucleovision Imaging Workstation (Nucleotech Corporation, San Carlos, CA). Immunolabeling: Immunolabeling was performed on frozen sections using immunofluorescent methods previously described [9,21,22]. Sections were thawed, immersed in 4% formaldehyde for min at room temperature to improve adherence, rinsed, treated with 1-2% NaBH 4 for 1-2 min at room temperature to reduce non-specific autofluorescence, and rinsed again. Non-specific labeling was blocked in 10% normal goat serum plus 5% bovine serum albumin plus 0.5-1% fish gelatin plus 0.1% Triton X-100 in PBS (blocker). For experiments utilizing the goat anti-chat antibody, blocking was performed using 2% normal donkey serum plus 5% bovine serum albumin plus 0.5-1% fish gelatin plus 0.1% Triton X- 100 in PBS. Excess blocker was removed and primary antibody was applied overnight to 2 days at 4 C. For doublelabeling experiments, a combination of primary antibodies was applied simultaneously. Sections were rinsed, blocked for 30 min, and secondary antibody was applied for 45 min at room temperature. For double-labeling experiments, an appropriate combination of secondary antisera was applied simultaneously. Sections were rinsed, coverslipped in a fade-retardant mounting medium (DABCO, or Vectashield, Vector Labs, Burlingame, CA) [23], and examined. To confirm the specificity of immunolabeling methods, sections were processed in the absence of primary antibodies or by substituting normal rabbit serum for rabbit polyclonal primary antisera. These treatments eliminated immunolabeling, indicating that labeling was specific. Bleedthrough between the Cy3 and AlexaFluor 488 channels was assessed in sections treated with a single primary antibody and a combination of Cy3 and AlexaFluor 488- conjugated secondary antibodies. Only the channel that corresponded to the primary antibody showed labeling, as appropriate. All antibodies and antisera were diluted in the appropriate blocker solution. Imaging: Imaging methods were as previously described [9,21,22]. Greyscale images of immunofluorescent labeling were digitized directly from an Olympus IX70 microscope, using frame-averaging to reduce noise (24-36 frames/image). Image scale was calibrated and image brightness and contrast was adjusted to highlight specific immunolabeling, if necessary. Morphometric measurements were made from the scaled images using the functions of the Scion Image program (Scion Corporation, Frederick, MD). To assess colocalization of VAMP with other markers, matching images of labeling in the Cy3 and AlexaFluor 488 channels were captured, imported into Adobe Photoshop 5.0 (Adobe Systems, Inc., Mountain View, CA), and pseudocolored red or green. Color-coded images were superimposed to compare localization of VAMP with other markers. Areas of colocalization in overlay images appeared yellow to orange, single labeling appeared either red or green. Colocalization studies were performed using 40x (N.A., 1.25) or 63x (N.A., 1.32) objective lenses. Some VAMP-1-immunoreactive cells were imaged by confocal microscopy using Leica TCS SP2 or Bio-Rad Radiance 2100 confocal microscopy systems. Stacks of serial optical sections (0.3 µm thickness) of VAMP-1 immunoreactive cells in retinal cryosections were collected and projected into a single image plane to visualize dendritic arbors. Bleedthrough of signals between fluorescence channels in the confocal microscope was eliminated by adjusting laser power and detector sensitivity or by sequentially imaging each fluorescent channel. Figure 4. VAMP-2 is expressed at conventional synapses in the mouse retina. Double-labeling for VAMP-2 and synapsin I (SYN I), a marker for conventional synapses (Mandell et al., 1990). Labeling for VAMP-2 is prominent in synaptic terminals in both the OPL and IPL. Strong labeling for synapsin I is present in conventional synapses in the IPL and sparsely distributed conventional synapses in the OPL (arrows), which also show labeling for VAMP-2. Synapsin I labeling is absent from the ribbon synapse-containing photoreceptor terminals and a set of large terminals along the inner border of the IPL corresponding to the rod bipolar cell terminals (small arrowheads). Weak labeling for VAMP- 2 and synapsin I is also visible in the cell bodies of some amacrine cells (a) and presumptive displaced amacrine cells (da). Scale bars represent 20 µm. 677
6 To define the precise location of the VAMP-1 immunoreactive plexus in the mid-ipl, the distance from the INL/IPL border to the top and the bottom of the VAMP-1 plexus was measured and compared to the total depth of the IPL measured as the distance from the INL/IPL border to the IPL/GCL border in the same image. The result was expressed as a percentage of the total IPL depth, defining the INL/IPL border as 0% and the IPL/GCL border as 100% of IPL depth, respectively. Twenty-four images from the central to mid-peripheral regions of four different animals were analyzed. Results were expressed as mean ± standard error of the mean. RESULTS Isoform specificity of VAMP antibodies: The specificity of the VAMP-1 and VAMP-2 antibodies was confirmed by immunoblotting (Figure 1) as reported previously [9]. Both VAMP-2 antibodies produced strong labeling of the expected band at about 18 kda in both retina and brain membrane homogenates. The VAMP-1 antibody recognized the expected single band at about 18 kda in brain membrane homogenates. However, only very faint labeling of the band was seen in the retinal membrane homogenates, consistent with the sparse distribution of VAMP-1 in the retina (see below). In addition, pre-incubation of the VAMP-1 antiserum with the immunogen peptide eliminated immunolabeling on retinal sections as appropriate (data not shown). To further characterize the isoform selectivity of the VAMP-1 and VAMP-2 antisera, conservation of the N-terminus amino acid sequences that the antisera recognize was compared across mouse VAMP isoforms in the (Entrez database). Sequences were aligned using the ClustalW version 1.8 multiple sequence alignment program. The VAMP-1 epitope (amino acids 2-14 in the N-terminus) is poorly conserved in VAMP-2 (7.7% homology) and other VAMP isoforms (minimum homology, 0%, VAMP-3 and VAMP-5; maximum homology, 30.8%, VAMP-7). The epitope recognized by the polyclonal VAMP-2 antiserum (amino acids 2-17 in the N- terminus) is poorly conserved in VAMP-1 (18.8% homology) and other VAMP isoforms (minimum homology, 0%, VAMP- 4 and VAMP-5; maximum homology: 25%, VAMP-7). The specific N-terminus amino acid sequence recognized by the monoclonal VAMP-2 antibody has not been characterized [14]. Together, these immunoblotting, pre-adsorption, and sequence analyses indicate that the VAMP-1 and VAMP-2 antibodies were isoform-specific, confirming previous reports [9,14]. VAMP-1 and VAMP-2 are differentially distributed.: Labeling for VAMP-1 and VAMP-2 showed distinct distributions in the mouse retina (Figure 2). VAMP-2 was the predominant isoform in the retina (Figure 2A). VAMP-2 was associated with both ribbon and conventional synapses. The ribbon synapses of the rod and cone photoreceptors in the outer plexiform layer (OPL) showed very strong labeling for VAMP-2 as shown by colocalization with VGLUT1, a marker for the terminals of both rods and cones (Figure 3A-C). Double-labeling for VAMP-2 and VGLUT1 to specifically visualize the terminals of all bipolar cell types or PKC to visualize rod bipolar cells [24-26] confirmed that VAMP-2 also was present in the terminals of rod and cone bipolar cells (Figure 3D-I). Double-labeling for VAMP-2 in conjunction with the conventional synapse markers, synapsin I, glutamic acid decarboxylase 65 kda isoform (GAD-65), glycine, and calretinin showed that VAMP-2 also was broadly distributed among conventional synapses (Figure 4; synapsin I shown). There were no differences in labeling associated with retinal location or among the three mouse strains studied for VAMP-2. Figure 5. Weak VAMP-1-immunoreactivity in the OPL is associated with photoreceptor terminals. A-C: Double-labeling for VAMP-1 and VGLUT1, a marker for photoreceptor terminals in the OPL. Labeling for VAMP-1 colocalizes with labeling for VGLUT1, indicating that VAMP-1 labeling in the OPL is associated with photoreceptor terminals. D-F: Double-labeling for VAMP-1 and calbindin (CaBP), a marker for horizontal cells (H). The two labels do not colocalize. Labeling for VAMP-1 is located distal to CaBP labeling in the horizontal cell processes in the proximal OPL, consistent with expression of VAMP-1 in photoreceptor terminals rather than the dendrites of second-order neurons. Scale bars represent 10 µm. 678
7 The distribution of VAMP-1 labeling was much more restricted than VAMP-2 labeling (Figure 2B). Labeling for VAMP-1 in the synaptic layers was most prominent in the inner plexiform layer (IPL). Labeling for VAMP-1 in the IPL was present in puncta sparsely distributed throughout the layer, with a distinctive plexus in the mid-ipl, occupying 21.2 ± 1.0% of the width of the IPL, starting at a depth of 29.7 ± 0.8% and extending to a depth of 50.8 ± 1.4% of the IPL width (INL/IPL border defined as 0%; mean ± standard error of the mean). Weak labeling for VAMP-1 in the OPL (inset, Figure 2B) was specifically associated with rod and cone terminals, as shown by colocalization of VAMP-1 and VGLUT1 labeling (Figure 5A-C). VAMP-1 labeling in the OPL did not colocalize with labeling for calbindin (CaBP) a marker for horizontal cells and their processes (Figure 5D-F). Cell bodies showing VAMP-1 labeling were present in the ganglion cell layer (GCL) and also were found in the inner nuclear layer (INL) occasionally. Cells showing VAMP-1 labeling were present at all retinal eccentricities, although in this initial study their distribution across the retina was not studied in detail. Prominent labeling for VAMP-1 also was observed in bundles of ganglion cell axons along the inner border of the retina. There were no differences in VAMP-1 labeling associated with retinal location or among the three mouse strains. VAMP-1 is expressed in morphologically diverse cells, including subsets of ganglion cells: A distinctive feature of VAMP-1 labeling was the presence of sparsely distributed, intensely labeled cells. Cells labeled for VAMP-1 were morphologically heterogeneous (Figure 6). Most of these cells were located in the GCL, which in the mouse contains a very high proportion of displaced amacrine cells in addition to the ganglion cells [27]. A set of VAMP-1-immunoreactive cells lo- Figure 6. VAMP-1-immunoreactive cells show heterogeneous morphology. A-B: Large VAMP-1-immunoreactive cells in the GCL projecting to the VAMP-1 plexus in the mid-ipl. A: Two large VAMP-1 immunoreactive cells (*) in the GCL. Axons (Ax) arise from both cells, indicating that they are ganglion cells. The cell at the left has a single, large primary dendrite and branches (arrowheads) in the VAMP-1- immunoreactive plexus in the mid-ipl (identified with brackets). The cell at the right has more slender primary dendrites (arrowheads) that ramify in the proximal portion of the IPL. B: Another large VAMP-1-immunoreactive cell (*) in the GCL showing multiple primary dendrites and complex dendritic branching (arrowheads) in the VAMP-1 plexus in the mid-ipl (identified with brackets). C: A large VAMP-1-immunoreactive cell (*) in the inner nuclear layer (INL). These cells also showed one or two primary dendrites (arrowheads) projecting to the VAMP-1-immunoreactive plexus in the mid-ipl (identified with brackets) and typically had large pyriform cell bodies. D: A few large VAMP- 1-immunoreactive cells (*) in the INL exhibited a rounded soma. Another large VAMP-1-immunoreactive cell (arrow) with a large primary dendrite (arrowhead) is present in the GCL. E: A smaller VAMP-1-immunoreactive cell (*) in the GCL. A slender primary dendrite (arrowhead) can be seen extending into the proximal portion of the IPL. Small VAMP-1-immunoreactive cells typically had a rounded soma. A second labeled cell out of the plane of focus is visible at the left side of the panel (*). F: Example of another comparatively small, round cell in the GCL showing VAMP-1 labeling (*). Panels A and B were created by projecting four conventional fluorescent images taken at different focal planes into a single image using the maximum projection function of the NIH Image program. Scale bars represent 10 µm. 679
8 cated in the INL also was observed, but was encountered infrequently. The most prominent and distinctive set of cells that labeled for VAMP-1 was characterized by a large cell body that protruded into the IPL, an eccentrically placed nucleus, and one or more stout dendrites that extended to the VAMP-1 plexus in the mid-ipl (Figure 6A,B,D). Axons arose from the proximal pole of several of these cells, indicating that at least some cells with these characteristics were ganglion cells (Figure 6A). Confocal microscopy of several of these cells confirmed that the cells possessed axons and showed that their dendrites contributed directly to the VAMP-1 plexus in the mid-ipl (Figure 7). A set of large VAMP-1 immunoreactive cells with large cell bodies located in the INL also projected to the VAMP-1 plexus in the mid-ipl, but were much less numerous than the cells in the GCL (Figure 6C,D). A variety of other VAMP-1 immunoreactive cells also were present in the GCL (Figure 6A,E,F). These cells varied widely in size. Some possessed axons, indicating that they were ganglion cells (Figure 6A), but the possibility that some also were displaced amacrine cells could not be eliminated on the basis of VAMP- 1 labeling alone. The dendritic ramifications of most of these cells in the IPL was not clearly visible, but dendrites from some of the cells coursed in the proximal IPL (Figure 6A,E). Figure 7. Large VAMP-1 immunoreactive ganglion cells contribute to the VAMP-1 immunoreactive plexus in the mid-ipl. Confocal microscopy shows that the large VAMP-1 immunoreactive ganglion cells contribute to the VAMP-1 immunoreactive plexus in the midinner plexiform layer (IPL). Two dimensional projection of a large VAMP-1-immunoreactive ganglion cell (*) in the ganglion cell layer (GCL) constructed from 43 optical sections totaling 12.6 µm thickness. At the level of the mid-ipl the primary dendrite (arrow) splits and smaller branches showing VAMP-1-immunoreactive puncta (arrowheads) distribute in the VAMP-1 plexus in the mid-ipl (square bracket symbol). A fragment of the cell s axon (ax) is also visible, indicating the cell is a ganglion cell. Nearby, part of the cell body of another nearby VAMP-1 immunoreactive cell is visible in the GCL. Several labeled axons are visible in the nerve fiber layer (NFL). INL, inner nuclear layer. Scale bar represents 20 µm. 680 The morphological characteristics and presence of axons suggested that the VAMP-1 immunoreactive cells were ganglion cells. To further characterize the identity of the VAMP- 1-immunoreactive cells as ganglion or amacrine cells, double labeling was performed for VAMP-1 and two different ganglion cell markers, microtubule associated protein-1 (MAP- 1), a marker for ganglion cell dendrites and cell bodies [28], and Brn-3a, a transcription factor described as preferentially localized to nuclei of small ganglion cells [29]. Labeling for VAMP-1 and MAP-1 colocalized extensively. The large VAMP-1-immunoreactive cells in the GCL and INL that projected to the VAMP-1 plexus in the mid-ipl also showed MAP-1 labeling (Figure 8A-F), positively identifying these cells as ganglion cells. Other VAMP-1-immunoreactive cells in the GCL also showed MAP-1 labeling, indicating that VAMP-1 labeling was present in other types of ganglion cells as well (Figure 8G-I). Labeling for VAMP-1 and Brn-3a showed that Brn-3a levels varied appreciably (Figure 9). Many ganglion cells, positively identified by nuclear Brn- 3a labeling, did not show labeling for VAMP-1, indicating that VAMP-1 was not expressed by all ganglion cells. In the specific case of the large VAMP-1-immunoreactive ganglion cells projecting to the mid-ipl, some of these cells showed Brn-3a labeling (Figure 9A-C) while others did not (Figure 9D-F), indicating that Brn-3a levels differed even among morphologically similar ganglion cells. Small cells in the GCL that showed VAMP-1 labeling, but lacked Brn-3a labeling, also were observed (Figure 9D-F). However, these cells could not be designated as displaced amacrine rather than ganglion cells due to the variability in Brn-3a labeling among ganglion cells. VAMP-1 does not colocalize with other synaptic vesicle proteins or with markers for amacrine and bipolar cell processes and terminals in the IPL: Although it was clear that VAMP-1 labeling was present in the cell bodies of some types of ganglion cells, it remained uncertain whether the VAMP-1- positive puncta in the IPL arose from ganglion cell dendrites or the processes and terminals of amacrine or bipolar cells. Therefore, we examined colocalization of VAMP-1 labeling with labeling for several other synaptic vesicle proteins and neurochemical markers associated specifically with the processes and terminals of amacrine and bipolar cells (Figure 10, Figure 11). Labeling for VAMP-1 did not colocalize with labeling for VAMP-2 (Figure 10A-C), indicating that the two VAMP isoforms were expressed independently in the IPL. Labeling for VAMP-1 and synapsin I also did not colocalize (Figure 10D-F), indicating that the puncta labeled for VAMP-1 were not synapsin I-containing conventional synapses. Similarly, VAMP-1 labeling did not colocalize with labeling for SV2 (Figure 10G-I), a ubiquitous synaptic vesicle protein [30-32]. The localization of VAMP-1 to structures that did not contain VAMP-2, synapsin I, or SV2 suggested that VAMP-1 labeling in the IPL was not associated with synaptic vesicles in a presynaptic terminal. To further characterize whether the VAMP-1-positive puncta in the IPL arose from ganglion cell dendrites or the processes and terminals of amacrine or bipolar cells, we ex-
9 Figure 8. Many VAMP-1-immunoreactive cells are ganglion cells. Double labeling for VAMP-1 and the ganglion cell marker, MAP-1, confirms that many VAMP-1-immunoreactive cells are ganglion cells. A-C: Large VAMP-1-immunoreactive cells (*) with dendrites (arrows) projecting to the VAMP-1-immunoreactive plexus (square bracket symbol) in the mid-ipl show double-labeling for MAP-1, indicating these cells are ganglion cells. A bundle of axons (Ax) showing labeling for both proteins is also visible. Blood vessels (bv) show non-specific labeling. D-F: Large VAMP-1-immunoreactive cells (*) in the INL show double-labeling for MAP-1 in their cell bodies and dendrites (arrows), indicating that they are displaced ganglion cells. A VAMP-1-immunoreactive ganglion cell (GC) double-labeled for MAP-1 is visible in the GCL, but is slightly out of the plane of focus. G-I: Other VAMP-1-immunoreactive cells (*) also show double labeling for MAP-1 in their cell bodies and dendrites (arrow), indicating they are ganglion cells. Blood vessels (bv) show non-specific labeling. Scale bars represent 10 µm. 681
10 amined colocalization of VAMP-1 with several neurochemical markers for the processes and terminals of amacrine and bipolar cells (Figure 11). Labeling for VAMP-1 and GAD-65, a marker for GABAergic amacrine cell processes and terminals, did not colocalize (Figure 11A-C). However, the VAMP- 1 plexus in the mid-ipl did co-stratify with a broad band of GAD-65-immunoreactive terminals located between two narrow GAD-65-poor bands in the characteristic location of the OFF and ON cholinergic starburst amacrine cell plexes. Double-labeling for VAMP-1 and CHAT, a marker for the symmetrically organized cholinergic ON and OFF starburst amacrine cells [33-35], showed that the VAMP-1-immunoreactive plexus in the mid-ipl was located precisely between the ON and OFF cholinergic plexes (Figure 11D-F). Labeling for VAMP-1 also was not found in the processes and synapses of glycinergic amacrine cells, as labeling for VAMP-1 and glycine did not colocalize (Figure 11G-I). To assess whether VAMP-1 labeling was associated with bipolar cell terminals, double labeling for VAMP-1 and VGLUT1 was performed (Figure 11J-L). Labeling for VAMP-1 did not colocalize with VGLUT1 labeling, indicating that VAMP-1 was not present in bipolar cell terminals. However, bipolar cell terminals labeled for VGLUT1 were often closely apposed to VAMP-1- immunoreactive puncta in the IPL, suggesting that synaptic interactions may exist between bipolar cells and the processes of VAMP-1-immunoreactive cells. These results strongly suggest that VAMP-1 labeling was not present in the conventional synapses or processes of amacrine cells or the ribbon synapses of bipolar cells. Recently, a putative glutamatergic amacrine cell that ramifies in the mid-ipl has been identified in the rodent retina based on labeling for VGLUT3 [36,37]. Double labeling for VAMP-1 and VGLUT3 showed that the VGLUT3 cells costratified precisely with the VAMP-1 plexus in the mid-ipl (Figure 12). Terminals from the VGLUT3 cells often were closely associated with processes and puncta from the large VAMP-1-immunoreactive ganglion cells, suggesting potential synaptic interactions. DISCUSSION Both VAMP-1 and VAMP-2 are expressed in the mouse retina. As expected from previous studies indicating widespread expression of VAMP-2 in the retina [7-9] and brain [5,6], VAMP- 2 is the predominant isoform in the mouse retina. In contrast, VAMP-1 shows a much more restricted distribution, consistent with a previous report from salamander retina indicating the presence of a second VAMP isoform with limited distribution [9]. The distributions of VAMP-1 and VAMP-2 were not mutually exclusive. Labeling for both isoforms was detected in the OPL, which is dominated by the large synaptic terminals of the photoreceptors, although labeling for VAMP-2 was much more intense than labeling for VAMP-1. Co-expression Figure 9. Brn-3a labeling varies among ganglion cells. VAMP-1 and Brn-3a localize to separate cellular compartments: VAMP-1 is restricted to the cytoplasm; Brn-3a is restricted to the nucleus. When co-expressed the two markers are present in the same cell but show non-overlapping distributions within the cell. A-C: A large VAMP-1-immunoreactive ganglion cell (V1 GC) shows particularly strong Brn-3a labeling in its nucleus (n). A small VAMP-1-immunoreactive cell (arrow) that shows only weak Brn-3a labeling in its nucleus (n) is also visible. Several nearby ganglion cells identified by nuclei that are positive for Brn-3a (*) do not show VAMP-1 labeling. A second large VAMP-1-immunoreactive ganglion cell is present in the ganglion cell layer (GCL) at the right, but its nucleus is not visible. D-F: A large ganglion cell at the right (V1 GC) and a small cell at the left (arrow) show labeling for VAMP-1, but neither cell s nucleus (n) shows labeling for Brn-3a. Three nearby ganglion cells do show Brn-3a labeling in their nuclei (*), but do not show labeling for VAMP-1. Brackets denote the VAMP-1-immunoreactive band in the mid-ipl. Blood vessels (bv) show non-specific labeling. Scale bars represent 20 µm. 682
11 of multiple isoforms of a synaptic vesicle protein within a single synaptic terminal is consistent with previous reports of co-expression in the retina and elsewhere in the brain [21,31]. In contrast, VAMP-1 and VAMP-2 are distributed independently in the inner retina, with VAMP-1 potentially localizing to post-synaptic or non-synaptic structures in the IPL and being preferentially associated with ganglion cells (see below), and VAMP-2 being present in both ribbon and conventional Figure 10. VAMP-1 does not colocalize with other synaptic vesicle markers in the IPL. A-C: Double-labeling for VAMP-1 and VAMP-2. Punctate labeling for VAMP-1 is present (large arrowheads), particularly in a broad band located in the mid-ipl (identified with brackets). A cell in the ganglion cell layer (*) and its primary dendrite (small arrowhead) shows intense labeling for only VAMP-1. Labeling for VAMP-2 is present in terminals throughout the IPL. Puncta in the IPL show only single labeling for either VAMP-1 (large arrowheads) or for VAMP-2 (arrows). D-F: Double-labeling for VAMP-1 and synapsin I (SYN I) in the IPL. Puncta labeled for VAMP-1 (large arrowheads) are present, including a prominent band in the mid-ipl (identified with brackets). An intensely labeled cell (*) and its dendrites (small arrowheads) also are visible. Labeling for synapsin I is present in numerous conventional synaptic terminals throughout the IPL (arrows), but does not colocalize with VAMP-1 labeling. G-I: Double-labeling for VAMP-1 and synaptic vesicle protein 2 (SV2), a ubiquitous synaptic vesicle marker. Puncta labeled for VAMP-1 (large arrowheads) are present in the IPL, including a prominent band located in the mid-ipl (identified with brackets). Two intensely labeled cells (large *), their dendrites (small arrowheads) and a bundle of axons (ax) are also visible. The cell at the right projects to the VAMP-1 plexus located in the mid-ipl (identified with brackets). Labeling for SV2 is present in numerous synaptic terminals throughout the IPL (arrows), including the large rod bipolar cell terminals along the inner margin of the IPL (small *). The two markers do not colocalize. Scale bars represent 10 µm. 683
12 Figure 11. VAMP-1 does not colocalize with markers for amacrine or bipolar cells and terminals. A-C: Double-labeling for VAMP-1 and GAD-65 (GAD), a marker for GABAergic amacrine cell terminals. VAMP-1 (arrowheads) and GAD-65 (arrows) label different populations of puncta in the IPL. The VAMP-1-immunoreactive plexus in the mid-ipl (square bracket symbol) costratifies with a GAD-65-immunoreactive plexus located between the ON and OFF cholinergic plexes (small arrowheads labeled on and off). Two VAMP-1-immunoreactive ganglion cells (*) are visible in the GCL. Blood vessels (bv) show non-specific labeling. D-F: Double-labeling for VAMP-1 and choline acetyltransferase (CHAT), a marker for the cholinergic starburst amacrine cells (A) and their processes. The VAMP-1-immunoreactive plexus in the mid-ipl (square bracket symbol) is located between the ON and OFF cholinergic plexuses (small arrowheads labeled on and off). Two VAMP-1-immunoreactive cells (*) and their primary dendrites (arrows) are visible in the GCL. G-I: Confocal microscopic image (single optical section, 0.3 µm thickness) of double labeling for VAMP-1 and glycine (GLY), a marker for many amacrine and displaced amacrine cells (A and *, respectively). Similar to GAD-65, VAMP-1 (arrowheads) and GLY (arrows) labels do not colocalize in the IPL. Numerous amacrine cells (A) show strong labeling for GLY in their som A presumptive displaced amacrine cell (*) shows weak labeling for GLY. J-L: Double-labeling for VAMP-1 and VGLUT1, a marker for bipolar cell terminals in the IPL. Puncta in the IPL showing VAMP-1 labeling (arrowheads) do not colocalize with the synaptic terminals of bipolar cells labeled for VGLUT1 (arrows). A large VAMP-1-immunoreactive cell (*) is visible in the GCL. A second, smaller VAMP-1-immunoreactive cell body is visible to its right. Scale bars represent 10 µm. 684
13 synapses. As noted in the introduction, another core protein, SNAP-25, also is found in both types of synapses, whereas individual syntaxin isoforms are found only in ribbon or conventional synapses. The reason for these differences in degree of differential distribution is not clear. There are similarities and differences in the distributions of VAMP-1 and VAMP-2 in the mouse retina compared to the salamander retina, the only other species for which the retinal distribution of VAMP isoforms has been reported [9]. The widespread expression of VAMP-2 among conventional and ribbon synapses in both synaptic layers is similar in the mouse and salamander retinas. Both species also show restricted expression of a second VAMP isoform in the retina, however, the distribution of this second isoform differs considerably between the two species. In the mouse retina, the second isoform is VAMP-1, which is associated particularly with ganglion cells. In contrast, the second VAMP isoform, presumed to be the salamander homologue of VAMP-1, is preferentially localized to amacrine cells and their synaptic terminals in the IPL. Thus, VAMP-1 may have a role at conventional synapses in the salamander retina, but not in the mouse retina. Identity of cells showing somatic VAMP-1 labeling: Although labeling for VAMP-1 is restricted to comparatively few cells in the mouse retina, their morphological diversity indicates that VAMP-1 is present in multiple cell types. Labeling for VAMP-1 is particularly associated with ganglion cells. Many VAMP-1 immunoreactive cells have large cell bodies and axons and label for the ganglion cell markers MAP-1 [28] and Brn-3a [29], clearly identifying them as ganglion cells. VAMP-1 labeling is not expressed ubiquitously by ganglion cells, however, as many Brn-3a-positive cells do not show VAMP-1 labeling. It also is not clear currently whether somatic labeling for VAMP-1 is restricted exclusively to ganglion cells. Some of the cells in the GCL that showed VAMP- 1 labeling did not show labeling for ganglion cell markers, suggesting that some displaced amacrine cells could express VAMP-1. However, VAMP-1 labeling did not colocalize with labeling for the displaced amacrine cell markers CHAT, GAD- 65, or glycine in the GCL. Similarly, there is no evidence that somatic VAMP-1 labeling is associated with any conventionally placed amacrine cells located in the INL. The large VAMP-1-immunoreactive cells projecting to the mid-ipl showed morphological characteristics suggesting that they may comprise a distinct ganglion cell type. These cells all showed a large soma and a conserved dendritic organization, projecting to the VAMP-1 immunoreactive plexus in mid- IPL located between the plexuses of the ON and OFF center starburst amacrine cells as revealed by CHAT labeling. A distinctive, conserved organization of dendritic structure in the IPL is characteristic of specific ganglion cell types and corresponds closely to the physiology of the cell type [38-43]. In addition, cells with these morphological characteristics were observed at all retinal eccentricities as was the stratum of VAMP-1-immunoreactive puncta in the IPL, suggesting that these cells are distributed in a manner that provides complete dendritic coverage of the retina, although this would need to be confirmed in further studies. The displaced ganglion cells showing VAMP-1-immunoreactivity may belong to the same Figure 12. Large VAMP-1-immunoreactive ganglion cells co-stratify with VGLUT3-immunoreactive amacrine cells. The large VAMP-1- immunoreactive ganglion cells co-stratify with VGLUT3-immunoreactive amacrine cells. A: A large VAMP-1-immunoreactive ganglion cell (*) projects to the plexus in the mid-ipl (square bracket symbol). B: The processes of VGLUT3 amacrine cells (A) form a dense plexus that co-stratifies with the VAMP-1 plexus in the mid-ipl (square bracket symbol). C: Overlay of A and B. The VAMP-1 and VGLUT3 plexes in the mid-ipl co-stratify precisely (square bracket symbol). Terminals from the VGLUT3 cells are often seen in close proximity to the VAMP-1- immunoreactive puncta and the dendrites of the large VAMP-1-immunoreactive ganglion cell, suggesting potential synaptic interactions. Scale bar represents 10 µm. 685
14 cell type as the large VAMP-1-immunoreactive ganglion cells in the GCL as they show similar dendritic organization and somatic size characteristics. Ganglion cells in the mouse retina include several morphological and physiological types [44-51]. Ganglion cells with ON or OFF center receptive field organization similar to cat X- and Y-ganglion cells, and ON and ON-OFF directionallyselective responses are all present in the mouse retina [47,49]. The morphologies of most of these functional cell types, however, are currently unknown. The large VAMP-1-immunoreactive ganglion cells projecting to the mid-ipl clearly do not correspond to the large alpha ganglion cells that ramify narrowly in either sublamina a or sublamina b of the IPL [42,46,51]. The large VAMP-1-immunoreactive ganglion cells projecting to the mid-ipl also are unlikely to correspond to either the directionally-selective ON or ON-OFF ganglion cells reported in the mouse retina by Yoshida et al. [49]. Directionally-selective ON and ON-OFF cells, which are best characterized in the rabbit retina, have dendrites that co-stratify with the processes of the cholinergic starburst amacrine cells and receive direct input from the starburst cells [40,41,43,52,53], an organization very different from that of the large VAMP-1-immunoreactive ganglion cells that project to the area of the IPL lying between the cholinergic plexes of the starburst cells. On the basis of somatic size, the large VAMP-1-immunoreactive ganglion and displaced ganglion cells projecting to the mid-ipl would be classified as Type I cells using the classification system established for mouse ganglion cells by Doi et al. [48] and as Group A retinal ganglion cells (RG A ) using the system employed by Sun et al. [50]. However, none of the conventionally-placed or displaced ganglion cell types identified by Doi et al. [48] or Sun et al. [50], clearly correspond to the large VAMP-1-immunoreactive cells projecting to the mid-ipl observed in this study. Although the physiology and synaptology of the large VAMP-1-immunoreactive ganglion cells projecting to the mid- IPL are not known, predictions can be made about the physiology of the cells and their inputs. Co-stratification of the dendrites of the large VAMP-1-immunoreactive ganglion cells with a broad band of GAD-65 immunoreactive terminals suggests that the cells may receive major inhibitory GABAergic inputs from one or more amacrine cell types containing GAD- 65. The recently identified VGLUT3 cell, a putative glutamatergic amacrine cell [36,37], is also a candidate to provide input to the large VAMP-1-immunoreactive ganglion cells as the two cell types co-stratify in the mid-ipl and show close apposition in the mid-ipl. The cells also may receive input directly from cone bipolar cells based on the close apposition of some bipolar cell terminals to VAMP-1 immunoreactive puncta within the mid-ipl. The broad band of large VAMP-1- immunoreactive ganglion cell dendrites through the mid-ipl positions these cells to receive both ON and OFF center inputs, making them excellent candidates to be an ON-OFF ganglion cell type. However, the large VAMP-1-immunoreactive ganglion cells ramify between, but do not overlap with, the paired ON and OFF center strata in the IPL formed by the 686 starburst amacrine cells. This makes it unlikely that they receive significant direct input from starburst amacrine cells, known to be involved in ganglion cell responses to moving stimuli in the rabbit retina [40,41,43,52-55] and critical for directionally-selective ganglion cell responses in the mouse retina [49]. Differential distribution of VAMP-1 and VAMP-2 in the IPL: The two VAMP isoforms investigated in the current study, VAMP-1 and VAMP-2, have been associated specifically with synaptic vesicles [4,14]. It is clear that VAMP-2 is widely distributed and is the predominant isoform in the conventional and ribbon synapses of the mouse IPL. In contrast, VAMP-1 shows only a very restricted distribution in the IPL and our studies indicate that it may well be associated with post-synaptic or non-synaptic structures, rather than presynaptic terminals. The finding that labeling for VAMP-1 in the IPL did not colocalize with SV2, synapsin I, or VAMP-2 provides compelling evidence against VAMP-1 in the mouse IPL being associated with synaptic vesicles involved in neurotransmitter release. A further indication that VAMP-1 is not expressed in presynaptic terminals in the mouse IPL comes from the failure of VAMP-1 to colocalize with GAD-65, CHAT, or glycine, indicating that VAMP-1 is absent from GABAergic, glycinergic, and cholinergic amacrine cell synapses, which account for the vast majority of amacrine and displaced amacrine cell synapses. Similarly, VAMP-1 does not colocalize with VGLUT1, indicating that VAMP-1 also is not associated with the ribbon synapses of bipolar cells. An association of VAMP-1 with some sort of atypical vesicular synapse utilizing other neurotransmitters or neuromodulators that do not require other common synaptic vesicle proteins for release is unlikely, as there is no direct evidence for such a synapse to our knowledge. These findings, together with the finding that VAMP-1 is highly associated with the cell bodies of ganglion cells, strongly suggest that VAMP-1 labeling in the IPL is associated with ganglion cell dendrites. It is not clear currently what function VAMP-1 might serve outside the presynaptic terminal, however, VAMPs are known to mediate membrane fusion events involved in intracellular trafficking and axonal growth in addition to transmitter release [56-59] and VAMP-1 specifically has been suggested to be involved in vesicular trafficking in non-neural tissues as well [60]. An intriguing possibility is that VAMP-1 may be involved in rapid recycling of neurotransmitter receptors to and from the plasma membrane, which appears to involve membrane fusion and is sensitive to toxins that disrupt vesicular fusion [61,62]. It is not clear, however, why VAMP-1 should be restricted to such a small, specific subset of puncta in the IPL, rather than being more broadly distributed. ACKNOWLEDGEMENTS Supported by: VRSG, LGIA, PEER, and GEAR Awards from the University of Houston and College of Optometry (DMS); a student VRSG Award from the College of Optometry (MMW); National Institutes of Health grant number EY06671 (LJF); and National Institutes of Health grant numbers P30
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure S1. Generation of a synaptobrevin2-mrfp knock-in mouse. (a) Targeting strategy of Syb2-mRFP knock-in mouse leaving the synaptobrevin2 gene locus intact except
SUPPLEMENTARY FIGURES Supplementary Figure S1. Generation of a synaptobrevin2-mrfp knock-in mouse. (a) Targeting strategy of Syb2-mRFP knock-in mouse leaving the synaptobrevin2 gene locus intact except
2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non
 Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non 2. Plating density: sparse vs. confluent 3.
Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non 2. Plating density: sparse vs. confluent 3.
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney
 1 Supplementary text and data for: 2 3 4 5 Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney Authors: David A. D. Munro 1*, Peter Hohenstein 2, and Jamie A.
1 Supplementary text and data for: 2 3 4 5 Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney Authors: David A. D. Munro 1*, Peter Hohenstein 2, and Jamie A.
A guide to selecting control, diluent and blocking reagents
 Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
A guide to selecting control, diluent and blocking reagents
 Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Supplementary Figure 1 (related to Figure 2) Activation of microglia at early stages after light lesion. In the unlesioned retina, resident microglia
 Supplementary Figure 1 (related to Figure 2) Activation of microglia at early stages after light lesion. In the unlesioned retina, resident microglia labeled with the specific marker 4C4 (magenta) are
Supplementary Figure 1 (related to Figure 2) Activation of microglia at early stages after light lesion. In the unlesioned retina, resident microglia labeled with the specific marker 4C4 (magenta) are
Muscarinic Acetylcholine Receptor Localization and Activation Effects on Ganglion Response Properties
 Visual Neurophysiology Muscarinic Acetylcholine Receptor Localization and Activation Effects on Ganglion Response Properties Christianne E. Strang, 1 Jordan M. Renna, 1 Franklin R. Amthor, 2 and Kent T.
Visual Neurophysiology Muscarinic Acetylcholine Receptor Localization and Activation Effects on Ganglion Response Properties Christianne E. Strang, 1 Jordan M. Renna, 1 Franklin R. Amthor, 2 and Kent T.
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
DCLK-immunopositive. Bars, 100 µm for B, 50 µm for C.
 Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Axol Guide to Performing Immunocytochemistry (ICC) Application Protocol Version 2.0
 Axol Guide to Performing Immunocytochemistry (ICC) Application Protocol Version 2.0 Table of Contents General ICC Protocol 3 Synaptic Marker ICC Protocol 5 Recommended Markers 6 Technical Support 7 2 General
Axol Guide to Performing Immunocytochemistry (ICC) Application Protocol Version 2.0 Table of Contents General ICC Protocol 3 Synaptic Marker ICC Protocol 5 Recommended Markers 6 Technical Support 7 2 General
Figure S1 is related to Figure 1B, showing more details of outer segment of
 Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of
 Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Table S1. List of antibodies used including isotype controls, biotinylated. secondaries, and fluorophore conjugated tertiary antibodies.
 Table S1. List of antibodies used including isotype controls, biotinylated secondaries, and fluorophore conjugated tertiary antibodies. Antibody Description Distributor Catalogue number Working Concentration
Table S1. List of antibodies used including isotype controls, biotinylated secondaries, and fluorophore conjugated tertiary antibodies. Antibody Description Distributor Catalogue number Working Concentration
Displaced GAD65 amacrine cells of the guinea pig retina are morphologically diverse
 Visual Neuroscience ~2006!, 23, 931 939. Printed in the USA. Copyright 2006 Cambridge University Press 0952-5238006 $16.00 DOI: 10.10170S0952523806230293 Displaced GAD65 amacrine cells of the guinea pig
Visual Neuroscience ~2006!, 23, 931 939. Printed in the USA. Copyright 2006 Cambridge University Press 0952-5238006 $16.00 DOI: 10.10170S0952523806230293 Displaced GAD65 amacrine cells of the guinea pig
Visualizing Rhabdom Shedding in Whole Mounts of Photoreceptors from Limulus polyphemus
 Proceedings of The National Conference On Undergraduate Research (NCUR) 2006 The University of North Carolina at Asheville Asheville, North Carolina April 6 8, 2006 Visualizing Rhabdom Shedding in Whole
Proceedings of The National Conference On Undergraduate Research (NCUR) 2006 The University of North Carolina at Asheville Asheville, North Carolina April 6 8, 2006 Visualizing Rhabdom Shedding in Whole
Supplementary Methods. Li J.-Y. et al. Lewy bodies in grafted neurons in Parkinson s patients suggest host to. graft disease propagation
 1 Supplementary Methods Li J.-Y. et al. Lewy bodies in grafted neurons in Parkinson s patients suggest host to graft disease propagation Neural transplantation and clinical assessment Detailed information
1 Supplementary Methods Li J.-Y. et al. Lewy bodies in grafted neurons in Parkinson s patients suggest host to graft disease propagation Neural transplantation and clinical assessment Detailed information
Figures and figure supplements
 RESEARCH ARTICLE Figures and figure supplements Dendritic mitochondria reach stable positions during circuit development Michelle C Faits et al Faits et al. elife 2015;5:e11583. DOI: 10.7554/eLife.11583
RESEARCH ARTICLE Figures and figure supplements Dendritic mitochondria reach stable positions during circuit development Michelle C Faits et al Faits et al. elife 2015;5:e11583. DOI: 10.7554/eLife.11583
Materials and Methods Materials Required for Fixing, Embedding and Sectioning. OCT embedding matrix (Thermo Scientific, LAMB/OCT)
 Page 1 Introduction Tissue freezing and sectioning is a rapid method of generating tissue samples (cryosections) for histological analysis, and obviates the need for wax embedding. The method is popular
Page 1 Introduction Tissue freezing and sectioning is a rapid method of generating tissue samples (cryosections) for histological analysis, and obviates the need for wax embedding. The method is popular
Detection of protein expression
 Detection of protein expression by immunocytochemistry Dennis Brown, Ph. D. Program in Membrane Biology/Renal Unit MGH East Brown@receptor.mgh.harvard.edu http://membranebiology.mgh.harvard.edu Level of
Detection of protein expression by immunocytochemistry Dennis Brown, Ph. D. Program in Membrane Biology/Renal Unit MGH East Brown@receptor.mgh.harvard.edu http://membranebiology.mgh.harvard.edu Level of
Minicollagen cysteine-rich domains encode distinct modes of. Anja Tursch, Davide Mercadante, Jutta Tennigkeit, Frauke Gräter and Suat
 Supplementary Figure S1. Conformational dynamics of partially reduced CRDs. (A) Root mean square deviation (RMSD) and (B) radius of gyration (R G ) distributions for the N-CRD (green) and C-CRD (red) domains,
Supplementary Figure S1. Conformational dynamics of partially reduced CRDs. (A) Root mean square deviation (RMSD) and (B) radius of gyration (R G ) distributions for the N-CRD (green) and C-CRD (red) domains,
Antibodies used in this study Figure S1. Akt expression in neutrophils from WT and individual Akt isoform knockout mice
 ntibodies used in this study The anti β-actin monoclonal antibody (Sigma-ldrich, St. Louis, MO) was generated against a slightly modified human β-actin N-terminal peptide, c-sp-sp-sp-ile-la-la-leu-val-ile-
ntibodies used in this study The anti β-actin monoclonal antibody (Sigma-ldrich, St. Louis, MO) was generated against a slightly modified human β-actin N-terminal peptide, c-sp-sp-sp-ile-la-la-leu-val-ile-
DSCAM and DSCAML1 Function in Self-Avoidance in Multiple Cell Types in the Developing Mouse Retina
 Article DSCAM and DSCAML1 Function in Self-Avoidance in Multiple Cell Types in the Developing Mouse Retina Peter G. Fuerst, 1,6 Freyja Bruce, 2 Miao Tian, 3 Wei Wei, 5 Justin Elstrott, 5 Marla B. Feller,
Article DSCAM and DSCAML1 Function in Self-Avoidance in Multiple Cell Types in the Developing Mouse Retina Peter G. Fuerst, 1,6 Freyja Bruce, 2 Miao Tian, 3 Wei Wei, 5 Justin Elstrott, 5 Marla B. Feller,
Figure S1. Specificity of immunofluorescence staining in STC-1 cells. STC-1 cells
 Supplementary Figures and Tables Figure S1. Figure S1. Specificity of immunofluorescence staining in STC-1 cells. STC-1 cells were treated with donkey-anti rabbit antibody conjugated with Dylight488 or
Supplementary Figures and Tables Figure S1. Figure S1. Specificity of immunofluorescence staining in STC-1 cells. STC-1 cells were treated with donkey-anti rabbit antibody conjugated with Dylight488 or
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 sirna and shrna mediated depletion of ATP7A results in loss of melanosomal ATP7A staining. a-h, sirna mediated ATP7A depletion. Immunofluorescence microscopy (IFM) analysis of ATP7A
Supplementary Figure 1 sirna and shrna mediated depletion of ATP7A results in loss of melanosomal ATP7A staining. a-h, sirna mediated ATP7A depletion. Immunofluorescence microscopy (IFM) analysis of ATP7A
Immunofluorescence and phalloidin labeling of mammalian cells
 Immunofluorescence and phalloidin labeling of mammalian cells 2 Contents Materials for immunofluorescence and phalloidin labeling of mammalian cells...1 Immunofluorescence-labelling on cultivated adherent
Immunofluorescence and phalloidin labeling of mammalian cells 2 Contents Materials for immunofluorescence and phalloidin labeling of mammalian cells...1 Immunofluorescence-labelling on cultivated adherent
Association of Shank 1A Scaffolding Protein with Cone Photoreceptor Terminals in the Mammalian Retina
 Association of Shank 1A Scaffolding Protein with Cone Photoreceptor Terminals in the Mammalian Retina The MIT Faculty has made this article openly available. Please share how this access benefits you.
Association of Shank 1A Scaffolding Protein with Cone Photoreceptor Terminals in the Mammalian Retina The MIT Faculty has made this article openly available. Please share how this access benefits you.
Nr2e1 regulates retinal lamination and the development of Müller glia, S-cones, and glycineric amacrine cells during retinogenesis
 Corso-Díaz and Simpson Molecular Brain (2015) 8:37 DOI 10.1186/s13041-015-0126-x RESEARCH Nr2e1 regulates retinal lamination and the development of Müller glia, S-cones, and glycineric amacrine cells during
Corso-Díaz and Simpson Molecular Brain (2015) 8:37 DOI 10.1186/s13041-015-0126-x RESEARCH Nr2e1 regulates retinal lamination and the development of Müller glia, S-cones, and glycineric amacrine cells during
Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde
 Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Anti-MOUSE IgG (H&L) (GOAT) Antibody DyLight 488 Conjugated (Min X Bv Ch Gt GP Ham Hs Hu Rb Rt & Sh Serum Proteins)
 Anti-MOUSE IgG (H&L) (GOAT) Antibody DyLight 488 Conjugated (Min X Bv Ch Gt GP Ham Hs Hu Rb Rt & Sh Serum Proteins) - 610-141-121 Code: 610-141-121 Size: 100 µg Product Description: Anti-MOUSE IgG (H&L)
Anti-MOUSE IgG (H&L) (GOAT) Antibody DyLight 488 Conjugated (Min X Bv Ch Gt GP Ham Hs Hu Rb Rt & Sh Serum Proteins) - 610-141-121 Code: 610-141-121 Size: 100 µg Product Description: Anti-MOUSE IgG (H&L)
Supplemental Figure 1 Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical
 Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Kanaani et al., http://www.jcb.org/cgi/content/full/jcb.200912101/dc1 Figure S1. The K2 rabbit polyclonal antibody is specific for GAD67,
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Kanaani et al., http://www.jcb.org/cgi/content/full/jcb.200912101/dc1 Figure S1. The K2 rabbit polyclonal antibody is specific for GAD67,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature22047 Supplementary discussion Overall developmental timeline and brain regionalization in human whole-brain organoids We first defined the timeline of generation of broadly-defined cell
doi:10.1038/nature22047 Supplementary discussion Overall developmental timeline and brain regionalization in human whole-brain organoids We first defined the timeline of generation of broadly-defined cell
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES
 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Generation of inducible BICD2 knock-out mice. A) The mouse BICD2 locus and gene targeting constructs. To generate an inducible Bicd2
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Generation of inducible BICD2 knock-out mice. A) The mouse BICD2 locus and gene targeting constructs. To generate an inducible Bicd2
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation
 Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
Smooth Muscle-Specific Expression of ipla 2 β Participates in the Initiation and Early Progression of Vascular Inflammation and Neointima Formation Shu Liu 1, Zhongwen Xie 2, Qingwei Zhao 2, Huan Pang
GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts
 Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
SUPPLEMENTARY NOTE 3. Supplememtary Note 3, Wehr et al., Monitoring Regulated Protein-Protein Interactions Using Split-TEV 1
 SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
Nature Methods: doi: /nmeth Supplementary Figure 1. Retention of RNA with LabelX.
 Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
Methanol fixation allows better visualization of Kal7. To compare methods for
 Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Staining Techniques. Staining Techniques. There are many dyes. Histochemical Stains: chemical reactions. Feulgen reaction -DNA
 Staining Techniques There are many dyes. http://medinfo.ufl.edu/~dental/denhisto/stains.html Examples: Sudan black -Lipids Myelinated axons- blue ihcworld.com/imagegallery/displayimage.php?al... Weigert
Staining Techniques There are many dyes. http://medinfo.ufl.edu/~dental/denhisto/stains.html Examples: Sudan black -Lipids Myelinated axons- blue ihcworld.com/imagegallery/displayimage.php?al... Weigert
Figure S1. USP-46 is expressed in several tissues including the nervous system
 Supplemental Figure legends Figure S1. USP-46 is expressed in several tissues including the nervous system Transgenic animals expressing a transcriptional reporter (P::GFP) were imaged using epifluorescence
Supplemental Figure legends Figure S1. USP-46 is expressed in several tissues including the nervous system Transgenic animals expressing a transcriptional reporter (P::GFP) were imaged using epifluorescence
Hypoxyprobe -1 Pacific Blue Kit (HPI Catalog # HP15-XXX)
 Updated 2019 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Product Data Sheet for HP Pacific Blue M Picture: Pacific Blue anti pimonidazole mab on
Updated 2019 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Product Data Sheet for HP Pacific Blue M Picture: Pacific Blue anti pimonidazole mab on
Hypoxyprobe -1 Pacific Blue Kit (HPI Catalog # HP15-XXX)
 Updated 2018 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Product Data Sheet for HP Pacific Blue M Picture: Pacific Blue anti pimonidazole mab on
Updated 2018 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Product Data Sheet for HP Pacific Blue M Picture: Pacific Blue anti pimonidazole mab on
Molecular Techniques. 3 Goals in Molecular Biology. Nucleic Acids: DNA and RNA. Disclaimer Nucleic Acids Proteins
 Molecular Techniques Disclaimer Nucleic Acids Proteins Houpt, CMN, 9-30-11 3 Goals in Molecular Biology Identify All nucleic acids (and proteins) are chemically identical in aggregate - need to identify
Molecular Techniques Disclaimer Nucleic Acids Proteins Houpt, CMN, 9-30-11 3 Goals in Molecular Biology Identify All nucleic acids (and proteins) are chemically identical in aggregate - need to identify
Introduction to N-STORM
 Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Increased proliferation of late-born retinal progenitor cells by gestational lead exposure delays rod and bipolar cell differentiation
 Received 13 January 2016 Accepted 22 December 2016 Published 24 December 2016 Increased proliferation of late-born retinal progenitor cells by gestational lead exposure delays rod and bipolar cell differentiation
Received 13 January 2016 Accepted 22 December 2016 Published 24 December 2016 Increased proliferation of late-born retinal progenitor cells by gestational lead exposure delays rod and bipolar cell differentiation
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Mass spectrometry characterization of epoxide reactions.
 Supplementary Figure 1 Mass spectrometry characterization of epoxide reactions. (a) Degree of amine reactivity of bovine serum albumin (BSA) with epoxide molecules having different numbers of epoxide groups:
Supplementary Figure 1 Mass spectrometry characterization of epoxide reactions. (a) Degree of amine reactivity of bovine serum albumin (BSA) with epoxide molecules having different numbers of epoxide groups:
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
Panx2 expression modulates neuronal differentiation SUPPLEMENTAL DATA. Figure Legends
 Panx2 expression modulates neuronal differentiation SUPPLEMENTAL DATA Figure Legends Suppl. Fig. S1. Antigenic determinants of the Panx2 antibodies employed in this study. (A) Schematic of mouse Panx2
Panx2 expression modulates neuronal differentiation SUPPLEMENTAL DATA Figure Legends Suppl. Fig. S1. Antigenic determinants of the Panx2 antibodies employed in this study. (A) Schematic of mouse Panx2
NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE.
 NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE. D. R. Aguirre, N. DiMassa, Chrystal Johnson, H. Eran, R. Perez, C.V.R. Sharma, M.V.R. Sharma,
NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE. D. R. Aguirre, N. DiMassa, Chrystal Johnson, H. Eran, R. Perez, C.V.R. Sharma, M.V.R. Sharma,
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or
 Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
In vivo recording, forepaw denervation, and isolation of slices: Methods for mapping the forepaw/lower jaw border in anesthetized adult rat primary
 Supplementary Methods In vivo recording, forepaw denervation, and isolation of slices: Methods for mapping the forepaw/lower jaw border in anesthetized adult rat primary somatosensory cortex (S1), forepaw
Supplementary Methods In vivo recording, forepaw denervation, and isolation of slices: Methods for mapping the forepaw/lower jaw border in anesthetized adult rat primary somatosensory cortex (S1), forepaw
The Journal of Neuroscience. Primary Authors Jeremy N. Kay Harvard U., Department of MCB Research Associate in Sanes Lab University of San Francisco
 Retinal Ganglion Cells with Distinct Directional Preferences Differ in Molecular Identity, Structure, and Central Projections Jeremy N. Kay, Irina De la Huerta, In-Jung Kim, Yifeng Zhang, Masahito Yamagata,
Retinal Ganglion Cells with Distinct Directional Preferences Differ in Molecular Identity, Structure, and Central Projections Jeremy N. Kay, Irina De la Huerta, In-Jung Kim, Yifeng Zhang, Masahito Yamagata,
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and
 Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Supplementary Figure 1. α-synuclein is truncated in PD and LBD brains. Nature Structural & Molecular Biology: doi: /nsmb.
 Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Hypoxyprobe -1 ATTO RED 594 Kit (HPI Catalog # HP13-XXX)
 Updated 2019 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Picture: RED 594 dye linked anti pimonidazole mab on hypoxic mouse lung tissue from a
Updated 2019 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Picture: RED 594 dye linked anti pimonidazole mab on hypoxic mouse lung tissue from a
Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor
 SUPPLEMENTARY INFORMATION Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor Anna Turetsky 1,a, Eunha Kim 1,a, Rainer H. Kohler 1, Miles A. Miller 1, Ralph Weissleder 1,2,
SUPPLEMENTARY INFORMATION Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor Anna Turetsky 1,a, Eunha Kim 1,a, Rainer H. Kohler 1, Miles A. Miller 1, Ralph Weissleder 1,2,
Supplemental Materials and Methods
 Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
F4/80, CD11b, Gr-1, NK1.1, CD3, CD4, CD8 and CD19. A-antigen was detected with FITCconjugated
 SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
Matching neural morphology to molecular expression: Single cell injection following immunostaining
 Journal of Neurocytology 32, 245 251 (2003) Matching neural morphology to molecular expression: Single cell injection following immunostaining YEN-HONG KAO and PETER STERLING Department of Neuroscience,
Journal of Neurocytology 32, 245 251 (2003) Matching neural morphology to molecular expression: Single cell injection following immunostaining YEN-HONG KAO and PETER STERLING Department of Neuroscience,
Superresolution Pattern Recognition Reveals the Architectural Map of the
 Supplementary Information Superresolution Pattern Recognition Reveals the Architectural Map of the Ciliary Transition Zone T. Tony Yang a, Jimmy Su b, Won-Jing Wang c, Branch Craige d, George B. Witman
Supplementary Information Superresolution Pattern Recognition Reveals the Architectural Map of the Ciliary Transition Zone T. Tony Yang a, Jimmy Su b, Won-Jing Wang c, Branch Craige d, George B. Witman
to KLH (keyhole-limpet hemocyanin), corresponding to amino acid residues of
 1 EN-09-0410-REVISED VERSION 2 May 11 th 2009 3 Supplemental Data - Battista et al. 2009 4 Supplemental Materials and Methods 5 6 7 8 9 10 Seladin-1 antiserum In short, rabbits were immunized with the
1 EN-09-0410-REVISED VERSION 2 May 11 th 2009 3 Supplemental Data - Battista et al. 2009 4 Supplemental Materials and Methods 5 6 7 8 9 10 Seladin-1 antiserum In short, rabbits were immunized with the
Supplementary Material - Methods
 Novel Protein-Protein Interactions in the Schizophrenia interactome Supplementary Material - Methods Experimental validations of predicted interactions Table S1-1: Protein pairs that were validated by
Novel Protein-Protein Interactions in the Schizophrenia interactome Supplementary Material - Methods Experimental validations of predicted interactions Table S1-1: Protein pairs that were validated by
SANTA CRUZ BIOTECHNOLOGY, INC.
 TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
Supplementary Table 1. Primary antibodies used in this study.
 Supplementary Table 1. Primary antibodies used in this study. Antibodies Mouse monoclonal Antibody(Ab) Working dilution Oct4 1:2 Pax6 1:1 Company Notes 1 Santa Cruz Biotechnology Use only Cy3 2nd antibody
Supplementary Table 1. Primary antibodies used in this study. Antibodies Mouse monoclonal Antibody(Ab) Working dilution Oct4 1:2 Pax6 1:1 Company Notes 1 Santa Cruz Biotechnology Use only Cy3 2nd antibody
Online Supplement ALVEOLAR CELL SENESCENCE IN PATIENTS WITH PULMONARY EMPHYSEMA. Takao Tsuji, Kazutetsu Aoshiba, and Atsushi Nagai
 Online Supplement ALVEOLAR CELL SENESCENCE IN PATIENTS WITH PULMONARY EMPHYSEMA Takao Tsuji, Kazutetsu Aoshiba, and Atsushi Nagai MATERIALS AND METHODS Immunohistochemistry Deparaffinized tissue sections
Online Supplement ALVEOLAR CELL SENESCENCE IN PATIENTS WITH PULMONARY EMPHYSEMA Takao Tsuji, Kazutetsu Aoshiba, and Atsushi Nagai MATERIALS AND METHODS Immunohistochemistry Deparaffinized tissue sections
Immunostaining Protocols
 Immunostaining Protocols Lula L. Hilenski, Ph.D. Director Microscopy in Medicine Core Emory University Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on
Immunostaining Protocols Lula L. Hilenski, Ph.D. Director Microscopy in Medicine Core Emory University Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on
Supplementary Information
 Supplementary Information Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction Hideto Takahashi 1, Kei-ichi Katayama 2, Kazuhiro Sohya 3,4, Hiroyuki Miyamoto 4,5,
Supplementary Information Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction Hideto Takahashi 1, Kei-ichi Katayama 2, Kazuhiro Sohya 3,4, Hiroyuki Miyamoto 4,5,
Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex
 Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex 1.0. Introduction Immunofluorescence uses the recognition of cellular targets by fluorescent dyes or antigen-specific antibodies coupled
Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex 1.0. Introduction Immunofluorescence uses the recognition of cellular targets by fluorescent dyes or antigen-specific antibodies coupled
Hypoxyprobe -1 Green Kit Kit contents:
 Updated 2015 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe -1 Green Kit Kit contents: Solid pimonidazole HCl (Hypoxyprobe -1) FITC conjugated
Updated 2015 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Hypoxyprobe -1 Green Kit Kit contents: Solid pimonidazole HCl (Hypoxyprobe -1) FITC conjugated
Marilyn G. Rimando, Hao-Hsiang Wu, Yu-An Liu, Chien-Wei Lee, Shu-Wen Kuo, Yin-
 Supplementary Information Glucocorticoid receptor and Histone Deacetylase 6 mediate the differential effect of dexamethasone during osteogenesis of Mesenchymal stromal cells (MSCs) Marilyn G. Rimando,
Supplementary Information Glucocorticoid receptor and Histone Deacetylase 6 mediate the differential effect of dexamethasone during osteogenesis of Mesenchymal stromal cells (MSCs) Marilyn G. Rimando,
Western blot troubleshooting guide
 Specializing in Secondary Antibodies and Conjugates Western blot troubleshooting guide Optimize your Western blotting with Jackson ImmunoResearch Secondary antibodies Troubleshooting for better blots Western
Specializing in Secondary Antibodies and Conjugates Western blot troubleshooting guide Optimize your Western blotting with Jackson ImmunoResearch Secondary antibodies Troubleshooting for better blots Western
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl
 Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging
 QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Hypoxyprobe -1 CY 7 Kit (HPI Catalog # HP14-XXX)
 Updated 2017 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Picture: Cy7 Near Infrared dye linked anti pimonidazole mab on hypoxic mouse lung tissue
Updated 2017 1 PRODUCT INSERT Hypoxyprobe, Inc 121 Middlesex Turnpike Burlington, MA 01803 USA www.hypoxyprobe.com Picture: Cy7 Near Infrared dye linked anti pimonidazole mab on hypoxic mouse lung tissue
Histone H3 Methylation Antibody Panel Pack I - Active Genes Base Catalog # C Component Size Shipping Temperature
 Histone H3 Methylation Antibody Panel Pack I - Active Genes Base Catalog # PACK CONTENTS Component Size Shipping Temperature Upon Receipt Checklist 3K4D Histone H3K4me2 (H3K4 Dimethyl) Polyclonal Antibody
Histone H3 Methylation Antibody Panel Pack I - Active Genes Base Catalog # PACK CONTENTS Component Size Shipping Temperature Upon Receipt Checklist 3K4D Histone H3K4me2 (H3K4 Dimethyl) Polyclonal Antibody
Supplemental material and methods
 Supplemental material and methods Antibodies: Primary antibodies used for these stainings were 174/2 1 (migg1 against PV-1), PAL-E 2 (migg2a; Abcam, Cambridge, UK), anti-nrp-1 (monoclonal migg2a or polyclonal
Supplemental material and methods Antibodies: Primary antibodies used for these stainings were 174/2 1 (migg1 against PV-1), PAL-E 2 (migg2a; Abcam, Cambridge, UK), anti-nrp-1 (monoclonal migg2a or polyclonal
Contents. 11 The Use of Epitope Tags in Histochemistry References... 98
 Contents 1 Antibodies for Immunohistochemistry... 1 1.1 Structure of Antibodies... 2 1.2 Polyclonal Antibodies... 4 1.3 Mouse Monoclonal Antibodies... 4 1.4 Rabbit Monoclonal Antibodies... 5 1.5 Protein
Contents 1 Antibodies for Immunohistochemistry... 1 1.1 Structure of Antibodies... 2 1.2 Polyclonal Antibodies... 4 1.3 Mouse Monoclonal Antibodies... 4 1.4 Rabbit Monoclonal Antibodies... 5 1.5 Protein
supplementary information
 Figure S1 Distribution of heterokaryons in vermis and hemispheres of the cerebellum. Schematic dorsal view of an adult cerebellum with the vermis in the middle (red) flanked by the two hemispheres (grey).
Figure S1 Distribution of heterokaryons in vermis and hemispheres of the cerebellum. Schematic dorsal view of an adult cerebellum with the vermis in the middle (red) flanked by the two hemispheres (grey).
SOX2 antibody - Embryonic Stem Cell Marker (ab15830) datasheet
 -Embryonic Stem Cell Marker (ab15830) 1/8 ページ d Products: Stem Cells >> Germline Stem Cells >> Embryonic Germ Cells SOX2 antibody - Embryonic Stem Cell Marker (ab15830) datasheet Product Name Product type
-Embryonic Stem Cell Marker (ab15830) 1/8 ページ d Products: Stem Cells >> Germline Stem Cells >> Embryonic Germ Cells SOX2 antibody - Embryonic Stem Cell Marker (ab15830) datasheet Product Name Product type
THE BASICS OF IMMUNOHISTOCHEMISTRY
 THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry of cytochrome c and mitochondria.
 PROTOCOL ApoTrack Cytochrome c Apoptosis ICC Antibody Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSA07 Rev.1 DESCRIPTION ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry
PROTOCOL ApoTrack Cytochrome c Apoptosis ICC Antibody Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSA07 Rev.1 DESCRIPTION ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry
Supplementary material and methods
 Inhibitory effect of caffeic acid on ADP-induced thrombus formation and platelet activation involves mitogen-activated protein kinases Yu Lu 1,2,3,#, Quan Li 3,4,#, Yu-Ying Liu 3,4, Kai Sun 3,4, Jing-Yu
Inhibitory effect of caffeic acid on ADP-induced thrombus formation and platelet activation involves mitogen-activated protein kinases Yu Lu 1,2,3,#, Quan Li 3,4,#, Yu-Ying Liu 3,4, Kai Sun 3,4, Jing-Yu
Input. Pulldown GST. GST-Ahi1
 A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
How to run Alpha assay: How to setup an Alpha assay Make your own assay!
 How to run Alpha assay: How to setup an Alpha assay Make your own assay! 1 2009 PerkinElmer AlphaLISA kits - recommendations before starting the assay Samples: Phenol red and hemoglobin: choose AlphaLISA
How to run Alpha assay: How to setup an Alpha assay Make your own assay! 1 2009 PerkinElmer AlphaLISA kits - recommendations before starting the assay Samples: Phenol red and hemoglobin: choose AlphaLISA
Supplementary Figure 1. Retinogenesis during human embryonic development and in 3-D retinal cups (RCs) derived from hipsc. a, Main steps leading to
 Supplementary Figure 1. Retinogenesis during human embryonic development and in 3-D retinal cups (RCs) derived from hipsc. a, Main steps leading to the formation of the human retina in vivo. b, Diagram
Supplementary Figure 1. Retinogenesis during human embryonic development and in 3-D retinal cups (RCs) derived from hipsc. a, Main steps leading to the formation of the human retina in vivo. b, Diagram
WESTERN BLOT. BCH462- Practical
 WESTERN BLOT BCH462- Practical What is Antigen [Ag]? What is Antibody [Ab]? Immunoassay: is a test that uses the highly specific and selective antigen-antibody reactions forming antibody and antigen complexes
WESTERN BLOT BCH462- Practical What is Antigen [Ag]? What is Antibody [Ab]? Immunoassay: is a test that uses the highly specific and selective antigen-antibody reactions forming antibody and antigen complexes
INOS. Colorimetric Cell-Based ELISA Kit. Catalog #: OKAG00807
 INOS Colorimetric Cell-Based ELISA Kit Catalog #: OKAG00807 Please read the provided manual entirely prior to use as suggested experimental protocols may have changed. Research Purposes Only. Not Intended
INOS Colorimetric Cell-Based ELISA Kit Catalog #: OKAG00807 Please read the provided manual entirely prior to use as suggested experimental protocols may have changed. Research Purposes Only. Not Intended
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in
 Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
The Dot-Spot Test. a simple method to monitor immunoreagent activity and influence of fixation on antigen recognition. Aurion Newsletter 4
 Aurion Newsletter 4 The Dot-Spot Test a simple method to monitor immunoreagent activity and influence of fixation on antigen recognition Peter F.E.M. van de Plas AURION Costerweg 5 6702 AA Wageningen The
Aurion Newsletter 4 The Dot-Spot Test a simple method to monitor immunoreagent activity and influence of fixation on antigen recognition Peter F.E.M. van de Plas AURION Costerweg 5 6702 AA Wageningen The
SUPPLEMENTAL MATERIAL. Supplemental Methods:
 SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
Immunohistochemistry guide
 Immunohistochemistry guide overview immunohistochemistry Overview Immunohistochemistry is a laboratory technique utilized for the visual detection of antigens in tissue. When working with cells this technique
Immunohistochemistry guide overview immunohistochemistry Overview Immunohistochemistry is a laboratory technique utilized for the visual detection of antigens in tissue. When working with cells this technique
In-Gel Western Detection Using Near-Infrared Fluorescence
 In-Gel Western Detection Using Near-Infrared Fluorescence Developed for: Aerius, and Odyssey Family of Imagers Please refer to your manual to confirm that this protocol is appropriate for the applications
In-Gel Western Detection Using Near-Infrared Fluorescence Developed for: Aerius, and Odyssey Family of Imagers Please refer to your manual to confirm that this protocol is appropriate for the applications
Histone H3K27 Methylation Antibody Panel Pack Base Catalog # C Component Size Shipping Temperature
 Histone H3K27 Methylation Antibody Panel Pack Base Catalog # PACK CONTENTS Component Size Shipping Temperature Upon Receipt Checklist 3K27M Histone H3K27me1 (H3K27 Monomethyl) Polyclonal Antibody 25 µg
Histone H3K27 Methylation Antibody Panel Pack Base Catalog # PACK CONTENTS Component Size Shipping Temperature Upon Receipt Checklist 3K27M Histone H3K27me1 (H3K27 Monomethyl) Polyclonal Antibody 25 µg
In general, 8- to 10-week-old adult females were ovariectomized and rested for 10 days
 Animal injections and tissue processing In general, 8- to 10-week-old adult females were ovariectomized and rested for 10 days before they received any injections. Mice were injected with sesame seed oil
Animal injections and tissue processing In general, 8- to 10-week-old adult females were ovariectomized and rested for 10 days before they received any injections. Mice were injected with sesame seed oil
Mice TRAMP mice were maintained in a C57BL/6J background. Syngeneic UBI-GFP/BL6 mice were used for bone marrow engraftment. 2
 Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
Antibodies Chicken IgY polyclonal α-gfp antibodies were purchased from Abcam (Cambridge, MA) and were detected using α-chicken IgY-FITC or α-chicken-hrp (also purchased from Abcam). The CD31-PE, CD11b-PE,
