DNA Y structure: a versatile, multidimensional. single molecule assay
|
|
|
- Tamsyn Taylor
- 6 years ago
- Views:
Transcription
1 DNA Y structure: a versatile, multidimensional single molecule assay James T. Inman 1, Benjamin Y. Smith 1,2,, Michael A. Hall 1,2,, Robert A. Forties 1,2, Jing Jin 1,2,, James P. Sethna 1, Michelle D. Wang 1,2,* 1 Department of Physics, LASSP, Cornell University, Ithaca, NY Howard Hughes Medical Institute, Cornell University, Ithaca, NY S1
2 Figure S1. Figure S2. Figure S3. Figure S4. Figure S5. Figure S6. Figure S7. Figure S8. Figure S9. Figure S10. Figure S11. Figure S12. Figure S13. Figure S14. Construction of the Y structure. A dual optical trap setup with bright field and fluorescence illumination. Calculation of Y structure forces and extensions. Component of force along arms perpendicular to trunk. Generating and stretching long ssdna tethers. Fork fluctuations. Geometry of the Y structure used for theoretical modeling Force-extension curves of ssdna and dsdna. Energies to stretch ssdna and dsdna. Forces under constant end positions of the Y structure. Forces under a constant trunk force of the Y structure. Arm forces under a torsionally constrained trunk of the Y structure. Geometry of the Y structure under constant forces in all three branches. Critical force of the Y structure under constant forces. Supporting Information Video 1. Simultaneous stretching, unzipping, and fluorescence. Supporting Information Discussion: 1. Y structure construction 2. Quantum-dot labelled RNA polymerase 3. Instrument design and calibration 4. Data collection and analysis 5. Calculation of Y structure forces and extensions 6. Generation and manipulation of long ssdna 7. Mapping the TEC structure 8. Theoretical models of Y structure unzipping Supporting Information References S2
3 Figure S1. Construction of the Y structure. (1) Arm 1 DNA was cut from plasmid pmdw38 (sequence available upon request) and its 5 end was labeled with digoxigenin with a Klenow reaction. (2) Upper 1 (5'-/phos/GCA GTA CCG AGC TCA TCC AAT TCT ACA TGC CGC)and lower 1 (5'-/phos/GCC TTG CAC GTG ATT ACG AGA TAT CGA TGA TTG CG GCG GCA TGT AGA ATT GGA TGA GCT CGG TAC TGC ATC G) were annealed to form adapter 1. (3) Adapter 1 was ligated to arm 1 and the product was gel purified to remove un-ligated adapters. (4) Steps 1-3 were repeated for arm 2: upper 1 (5'-CGT TAC GTC ATT CTA TAC ACT GTA CAG) and lower 2 (5'- /phos/gtaac CTG TAC AGT GTA TAG AAT GAC GTA ACG CGC AAT CAT CGA TAT CTC GTA ATC ACG TGC AAG GC CTA). (5) Arm 1 and arm 2 were annealed via lower 1 and lower 2. (6) Trunk DNA was prepared by PCR from pmdw2 (sequence available upon request) using a 5 fluorescein tag on one of the primers. (7) Arms were ligated to trunk DNA. S3
4 Figure S2. A dual optical trap setup with bright field and fluorescence illumination. A 1064-nm Gaussian trapping beam is coupled to a polarization maintaining single mode fiber (not shown). After collimation, the beam is sent through a half-wave plate and beam splitter that controls the input power and ensures that the beam is linearly polarized. A second half-wave plate rotates the polarization and this partitions the power in the dual trap. The beam is recollimated to ~ 5 mm by an expansion telescope and elevated to the proper height by a periscope. To form the dual trap, the single Gaussian laser beam is split into two orthogonally polarized beams by a polarizing beam splitting cube (PBSC) in the Beam Splitting Box. One beam is reflected off of a mirror that is mounted on a tip/tilt piezo. This mirror is mapped to the back focal plane of the objective such that it controls Trap 1 s position while Trap 2 remains S4
5 fixed. The two beams are recombined by a second PBSC and expanded to ~ 10 mm by the mapping telescope. They are introduced into a Nikon TE200 microscope s imaging path and later to the trapping plane by a dichroic mirror. Upon exiting the condenser, the laser beams are reflected by a second dichroic mirror and again split by a PBSC. Each beam is detected by a quadrant photodiode (QPD). Bright field illumination is accomplished by 625-nm LED light introduced through the condenser lens. This light passes through the laser dichroic and the fluorescence cube set and is imaged by a cooled CCD. Fluorescence illumination is produced by a mercury arc lamp. The light is filtered and introduced into the illumination path by a fluorescence filter set optimized for quantum dots (excitation nm, emission 625 nm). S5
6 Figure S3. Calculation of Y structure forces and extensions. (a) The projection of the Y structure onto the xy plane. The xy position of the Y junction is determined by the force and location of the trapped beads. (b) The z location of the junction is determined by geometry. (c) The forces are required to sum to zero at the junction. S6
7 Figure S4. Component of force along arms perpendicular to trunk. The Y structure was unzipped under a constant trunk force. (a) Component of force along arms perpendicular to the trunk versus number of base pairs unzipped under no trunk force (black) and 10 pn trunk force (red). Theoretical predictions are shown for comparison. (b) Component of mean force along arms perpendicular to the trunk versus force along trunk (black). For each trunk force, component of the force along arms perpendicular to the trunk was averaged over the first 1500 bp unzipped. Theoretical prediction is shown in red. S7
8 Figure S5. Generating and stretching long ssdna tethers. (a) Cartoons depicting the steps to generate a long ssdna tether. The initial Y structure contained a trunk with only one strand of the trunk end attached to the coverslip surface. S8
9 The Y structure was then fully unzipped to release the other trunk strand from the surface. The remaining tether was composed of a dsdna segment that had been one of the original arms and a newly generated long stretch of ssdna that had been part of the original trunk dsdna. This tether was subsequently stretched to obtain a force-extension curve of the composite dsdna and ssdna. (b) Force versus extension of ssdna. The force-extension curve of the ssdna was obtained after removing the contribution of the dsdna from the measured force-extension of the composite DNA. The resulting ssdna force-extension (black) was fit to a modified freelyjointed chain model (solid red) at forces > 10 pn, yielding a persistence length of nm, a stretch modulus of 470 pn, and a contour length of 2055 nm. Below 10 pn, an extrapolation of the fit is shown. S9
10 Figure S6. Fluctuations of the unzipping fork in the presence of a transcription bubble. (upper) Measured unzipping force in the presence of a paused transcription elongation complex (TEC) (black) compared with the calculated unzipping force in the absence of a TEC (grey). (lower) Calculated fork fluctuations (standard deviation of the number of base pairs unzipped) near the paused TEC. All calculations were performed for a constant trunk force of 4 pn. S10
11 Figure S7. Geometry of the Y structure for theoretical modeling. The Y structure is confined to the xy plane and is symmetric about the trunk. The extension of each segment of DNA is indicated: l t for the dsdna trunk, l ss for ssdna in each arm, and l ds for dsdna in each arm. S11
12 Figure S8. Force-extension curves of ssdna and dsdna. Shown are extensions per nucleotide of ssdna (red) and per base pair of dsdna (blue) as determined by measured DNA elasticity parameters. See Eqs. (1) and (2). S12
13 Figure S9. Energies to stretch ssdna and dsdna. Shown are the energies needed to stretch one nucleotide of ssdna (red) and one base pair of dsdna (blue) to specified extension and force. S13
14 Figure S10. Forces under constant end positions of the Y structure. Results are shown for several values of positions. (a) Force along arms versus. (b) component of arms mean force versus. (c) component of arms mean force versus. (d) Number (#) of base pairs unzipped versus. S14
15 Figure S11. Arm forces under a constant trunk force of the Y structure. Results are shown for several values of the trunk forces. (a) Force along arms versus. (b) component of arms mean force versus. (c) component of arms mean force versus. (d) Number (#) of base pairs unzipped versus. S15
16 Figure S12. Arm forces under a torsionally constrained trunk of the Y structure. Results are shown for several values of the trunk forces. (a) Force along arms versus. (b) Number (#) of base pairs unzipped versus. S16
17 Figure S13. Geometry of the Y structure under constant forces in all three branches. The Y structure is confined to the xy plane and is symmetric about the trunk. Each arm consists of ssdna held at a constant force of magnitude at angle with respect to the trunk. S17
18 Figure S14. Critical force of the Y structure under constant force. For this calculation, the homopolymeric DNA trunk is assumed to a base pairing energy. (Top panel) The critical force, at which the Y structure unzips, is plotted as a function of the angle of the applied force. corresponds to 1D unzipping where there is no force on the trunk. As trunk force increases above 65 pn, trunk DNA is expected to undergo a B-S phase transition (shaded region) which our theory does not consider. (Bottom panel) The critical force is plotted as a function of the force along the trunk for a more direct comparison with our experimental results. S18
19 Supporting Information Video 1. Simultaneous stretching, unzipping, and fluorescence. This Y structure contained two arms of different lengths in order to make the two arms easily distinguishable, and a trunk with a paused transcription elongation complex (TEC) formed with an HA-tagged E. coli RNAP. The RNAP was subsequently labelled by anti-ha, which was then labeled by secondary-antibody coated quantum dots. The trunk DNA containing an RNAP was subsequently unzipped, resulting in a head-on collision of the TEC with the unzipping fork. The RNAP was found to be bound to the trunk DNA prior to encountering the unzipping fork. It was visualized by fluorescence and its precise location on the dsdna was determined by its force signature during unzipping. After the unzipping fork passed through the bound RNAP, the RNAP was found to be retained on the template strand (the shorter Y arm). (Top) The actual images and data showing the location of the RNAP (red, fluorescence images), the trapped microspheres (green, bright field images), and the real-time measured extensions of three branches of the Y structure (white lines) as the trunk DNA was unzipped through the bound RNAP. (Bottom) The force on the arms versus the number of base-pairs unzipping is plotted. S19
20 Supporting Information Discussion 1. Y structure construction The Y structure DNA was constructed from three distinct dsdna segments: two arms and the trunk (Fig. S1). The arms were made by restriction enzyme cuts from plasmid pmdw38 (sequence available upon request) for symmetric arms or from plasmid prl574 1 for asymmetric arms. A single restriction cut (XhoI or SphI) in this plasmid created an overhang that was subsequently filled in with either dig-dutp or bio-datp by Klenow polymerase (NEB) to provide specific attachment to anti-digoxigenin or streptavidin coated microspheres respectively. A second restriction cut (BstXI or BstEII) created an overhang for ligation to an annealed trunk adapter oligos to generate a long (>30 bp) overhang on each arm. The two trunk adaptor oligos from the two arms were complementary to each other and were subsequently annealed to form Y arms with a short trunk (~30 bp). The annealed adaptor oligos were designed to create an overhang for ligation to the full length trunk. Such a design is modular so that the trunk is interchangeable. Trunk DNA was made via PCR with a primer containing a 5 fluorescein for subsequent anchoring to an anti-fluorescein surface and then cut with a restriction enzyme (AlwNI) to provide the proper overhang for ligation to the Y arms. A fluorescein and antifluorescein attachment has been shown to be strong (dissociation constant ~ M 2 ) and has been previously used as a suitable single molecule linkage 3. We found the lifetime of the fluorescein anti-fluorescein attachment to be comparable to that of digoxigenin and antidigoxigenin attachment. S20
21 The torsionally constrained trunk was made by ligation to a torsion adapter at the end of the Y trunk. The torsional adapter was ~ 500 bp made via PCR from pmdw2 (sequence available upon request) with a 1:5 mixture of dttp:fluorescein-dutp to provide multiple attachment points in both strands. For the RNAP fluorescence experiments, an asymmetric Y structure was made with two arms having different lengths in order to determine to which strand RNAP remained bound after unzipping. This was accomplished by cutting the arms out of the plasmid prl574 with restriction enzymes to create a longer DNA (dig arm with SalI and BstEII; bio arm with SapI and BstXI). For the co-directional collision template, the trunk contained a T7A1 promoter with a transcription start site located at 1065 bp from the Y-junction. For the head-on collision template, the transcription start site was located at 1108 bp from the Y-junction. 2. Quantum-dot labelled RNA polymerase For RNA polymerase unzipping experiments, a transcription elongation complex was formed on the trunk DNA following a protocol similar to that previously established 1,4. Briefly, the 3.7 kbp trunk DNA (10 nm) was incubated with E. coli RNA polymerase (100 nm) for 30 minutes at 37 C in the transcription buffer (25 mm Tris-Cl ph 8.0, 100 mm KCl, 4 mm MgCl 2, 1 mm DTT, 0.4 mg/ml BSA, 7.5% glycerol, 50 μm ATP/GTP/CTP, 100 μm ApU, 1U/ L SUPERase-In). The S21
22 resulting trunk contained a transcription elongation complex paused at +20 bp from the promoter. The trunk DNA was then ligated to the short Y-arm. The RNAP was fluorescently labeled with quantum dots using standard antibody labeling techniques which has been demonstrated not to interfere with protein-dna binding 5. Briefly, single molecule Y-structure tethers were formed in a microscope flow cell. Purified HA-tagged RNAP 6,7 as labeled with primary antibody to HA expressed in mouse (Covance). Excess antibodies were washed out of the chamber PBS. Quantum dots coated with secondary antibodies (Invitrogen A-10195) were flowed in to bind to the primary antibodies. Excess quantum dots were washed out of the chamber PBS. 3. Instrument design and calibration The dual optical trapping setup was a built upon a Nikon TE200 microscope with fluorescence and bright field microscopy capabilities (Fig. S2). The dual trap was created from a single laser source (Spectra Physics J20, 5 W) that was split into two beams by a polarizing beam splitting cube (PBSC) into orthogonal linear polarizations. One of the polarized beams was steered by a mirror mounted on a tip-tilt piezo (MCL Nano-MTA) 8. Although the two beams were orthogonally polarized, some interference between the two beams still existed due to the use of a high NA objective, leading to some cross talk between the two traps 9. To minimize this, we inserted optical windows in one of the two beams so that the path length difference between the beams was set to longer than the coherence length of the laser (3 mm). The two beams S22
23 were recombined with another PBSC before entering a custom-built trapping port which reflected the beams into the microscope objective with a dichroic mirror. The traps were formed at the focus of a water immersion objection (Nikon MRD07602). The two beams were collected by the condenser and split by a PBSC. Each polarized beam was detected by a quadrant photodiode (Pacific Silicon Sensor QP50-6SD2) by back focal-plane interferometry to provide positions and forces along x and y (lateral), and z (axial) directions for each microsphere in its trap. The flow cell was mounted on a 3D piezo stage (MCL Nano-LPQ) to allow movement of the coverglass surface relative to the traps. Epi fluorescence was excited by a mercury arc lamp. The fluorescent cube set was designed for quantum dots with emission at 625 nm (Chroma 32214). Bright field illumination was accomplished with a red LED (625 nm, Thorlabs M625L2 and LEDD1B) which was transmitted by the 625 nm emission filter of the cube set. This allowed both the bright field and fluorescence images to be collected by the same cooled CCD (Hamamatsu ORCA-ER). To interlace the bright field and fluorescence images, the camera controller triggered the LED to turn on and off using a custom-built LED controller. The optical traps were calibrated using previously established methods with untethered polystyrene microspheres (489 ± 13 nm in diameter, Polysciences 09836). In brief, microsphere position calibration was carried out by scanning a surface-adhered microsphere through the trap via the 3D piezo stage to map the quadrant photodiode outputs as a function of the x, y, and z positions of the stage. Trap stiffness calibration was carried out using both the power S23
24 spectrum and the viscous drag methods. Briefly, for the power spectrum method, the x, y, and z positions of a trapped bead were measured and their power spectra were used to determine the trap stiffness along x, y, and z respectively. For the viscous drag method, the x, y, and z positions of a trapped bead were measured as the bead was moved through the solution at a constant speed along x, y, or z direction. The resulting viscous force versus bead displacement was then used to calculate the trap stiffness. The results from these two methods agreed within statistical uncertainty. For small displacements, the trap stiffness was determined to be 1.19 ± 0.04 pn nm -1 W -1 along x, 1.60 ± 0.17 pn nm -1 W -1 along y, and 0.31 ± 0.04 pn nm -1 W -1 along z for trap 1 and 1.36 ± 0.08 pn nm -1 W -1 along x, 1.27 ± 0.08 pn nm -1 W -1 along y, and 0.25 ± 0.04 pn nm -1 W -1 along z for trap 2, with power being that estimated for the sample plane. Maximum power used in the experiments was ~120 mw per trap at the sample plane. In all experiments on a Y structure, the microsphere displacement was limited to the linear trapping regime (< 100 nm displacement). Although the Y-structure may lie in any plane that is formed by its surface anchor and the two trapped microspheres, in order to reduce the effect of trap center drift due to laser heating of the objective, the Y structure was extended predominately in the xy plane with a small z component (Fig. S3). S24
25 4. Data collection and analysis Sample chambers were prepared using standard single molecule methods 13,14. Briefly, a chamber was coated with anti-fluorescein (Molecular Probes) at 20 μg/ml by incubation for 10 min and then the surface was blocked with 4 mg/ml casein. Subsequently, the Y structure at 10 pm was introduced into the sample chamber and incubated for 10 min. Finally, microspheres (489 ± 13 nm, Polysciences 09836) coated with anti-digoxigenin or streptavidin were sequentially incubated in the chamber for 10 min each. During an experiment, Y structures were identified as two microspheres in close proximity to each other undergoing constrained Brownian motion. These accounted for ~10% of the total tethers in the chamber. The two microspheres were readily separated into the two traps which were placed ~ 1 m apart and centered over the Y structure. In some cases, the two microspheres fell into the same trap. If this occurred, both microspheres were released and the process was repeated. It typically took 5-20 s to configure a Y structure for experiments. Custom software (LabVIEW 2010) was then used to automate several routines. First, prior to trapping a Y-structure, without microspheres in the trap, baseline data were recorded as the steered trap was scanned across the xy (lateral) plane. These baseline data were used to make corrections to experimental data. Second, the Y-structure anchoring point to the surface was centered between the two trapped microspheres by stretching the tether with the piezo stage using an algorithm similar to that previously described 10. Third, the height of the traps above the coverslip surface was determined by moving the coverslip towards the trapped S25
26 microspheres and detecting the z piezo position when the microspheres came into contact with the surface 12. Fourth, the Y-structure was then stretched and unzipped by moving both the steered trap and the piezo stage. A constant force on the trunk during unzipping experiments was maintained by feedback on the piezo stage and laser power at 100 Hz 10. The 3D location for the junction of the Y-structure was determined as the intersection of the lines of force from the microspheres positions. Thus the extensions of the three branches of the Y-structure were determined from the positions of the microspheres, the Y-structure anchoring point, and the Y-structure junction location. The forces on the arms were measured directly and the force on the trunk was determined by requiring the net force at the junction to be zero. Force and extension data for each arm were used for conversion to number of base pairs unzipped 11 (see Calculation of Y structure forces and extensions ). During fluorescence experiments, interlaced images of bright field and fluorescence were acquired by the CCD at 31.4 frames per second. To create an overlaid image such as those shown in Fig. 4b, a pair of bright field and fluorescence images were pseudo-colored and combined. To overlay the Y-structure configuration to indicate the three DNA segments, the 3D Y-structure configuration as determined from the optical trapping data was projected onto the xy plane and displayed as three white lines for the three DNA segments. The locations of the two microspheres were used as a reference to align the Y-structure to the images. S26
27 5. Calculation of Y structure forces and extensions During an experiment, the 3D locations and force vectors of the two trapped microspheres as well as the position of the coverslip surface were measured in real time. To fully characterize the Y structure, the force and extension of each segment of the Y structure as well as the Y structure geometry were calculated from the raw data collected by our dual trap instrument. Below is a description of this calculation. Below we refer to the coordinate system defined in Fig. S3. (1) The 3D location of the Y junction was determined. The xy location of the Y junction was located as the intersection of the lines of xy forces from the microspheres positions (Fig. S3a). The z coordinate of the junction was determined by the geometry defined by the height of the microspheres above the surface and the y position of the junction (Fig. S3b). (2) Once the position of the junction was known, the extension of each branch of the Y structure was determined as the distance between its two endpoints. (3) Finally, the force vectors on the arms were directly measured by the optical trap. The force on the trunk was determined by requiring that the net force at the junction to be zero (Fig. S3c). Thus, the force vector on the trunk was simply the opposite of the vector sum of the optical forces on the trapped microspheres. The number of base pairs unzipped was calculated from the force and extension measurements described above. First, the force and extension of each branch of the Y structure were measured under lower forces (< 15 pn) prior to unzipping. These were taken as initial S27
28 characterization of the Y structure. As the Y structure was unzipped, the extension in each arm at a given force increased beyond what could be accounted for by dsdna alone. The additional extension was attributed to ssdna. We used the modified freely-jointed-chain model of ssdna 10,15 to calculate the number of base pairs of ssdna in each arm. The ssdna in the arms was a measure of the number of base pairs unzipped. To achieve near base pair resolution, the resulting force versus number of base pairs unzipped curve was aligned to the corresponding theoretical curve discussed in the next section using a cross-correlation method we previously developed 14,16,17 To account for minor instrumental drift, trapping-bead size variations, and DNA linker variations, the alignment allowed for a small additive shift (~ 20 bp) and a multiplicative linear stretch (~ 3%). 6. Generation and manipulation of long ssdna ssdna is an important substrate, or intermediate, during replication, DNA repair and recombination, where long stretches of thousands of base pairs of ssdna are operated on by a variety of proteins. It has been experimentally challenging to generate and manipulate long ssdna of arbitrary sequence using an optical trap. Previous methods relying on DNA stretching require the application of high force (~ 65 pn) and/or chemical reagents 15,18,19 or the use of enzymatic reactions 20 to facilitate strand separation. Methods using DNA unzipping do not subject a DNA molecule to excessive forces but yield ssdna of complementary sequences that anneal upon force reduction 11,21. S28
29 Here, we demonstrate the use of the Y structure, first to generate ssdna of many kilo-base pairs of arbitrary sequence, and then to manipulate it from low to high forces. In order to generate ssdna, we used a Y-structure version with only one strand of its trunk end attached to the microscope coverslip (Fig. 1a). The dsdna trunk was then unzipped to completion using a method similar to that described in Fig. 1 and Fig. 2. Once the trunk was fully unzipped, one strand of the trunk remained attached to the coverslip and was composed of one dsdna arm and the ssdna of the trunk of the original Y structure (Fig. S5a). The other strand, and its associated arm, retracted to their trapped microsphere which was subsequently released into solution. The remaining tether was then stretched with one of the traps and its force-extension curve was measured. After removing the contribution to the force-extension from the dsdna arm 10, the force-extension curve of the ssdna was obtained (Fig. S5b). The force-extension of ssdna was well characterized by a modified freely-jointed chain model 15 at forces > 10 pn, yielding fit parameters in good agreement with those previously established 11,15. Below this force, the relation showed less well defined features, as a result of the formation of secondary structures in the ssdna at low forces 22, Mapping the TEC structure We found that when DNA containing a TEC was unzipped co-directionally with the direction of transcription, a force drop occurred a few base pairs before the expected location of the edge of the transcription bubble. We interpret this as a result of thermal fluctuations of the S29
30 unzipping fork. As DNA is unzipped, the unzipping fork is expected to fluctuate among multiple energy states, at rates much faster than the unzipping speed. Therefore, the measured fork position represents the mean value for the fork position 21,24. The extent of fluctuations is DNA sequence-dependent but is of the order of 5-10 base pairs (Fig. S6). As the fork approaches a DNA bubble, the fork s excursions away from the mean may encounter the DNA bubble. At this point, the fork will immediately open the entire bubble and become trapped in the much lower energy open state. Thus, the location of the start of a bubble will always be detected closer to the unzipping fork than the bubble s actual edge. The extent of the shift will be related to the local DNA sequence, the temperature, and the rate of unzipping. By contrast, the location of the end of the bubble can be determined with much more certainty. Therefore, when the region of force drop is used to determine the bubble size, the size is always over-estimated. 8. Theoretical models of Y structure unzipping We have extended a previous theoretical model of 1D DNA unzipping using equilibrium statistical mechanics methods 21,25-27 to model 2D DNA unzipping of a Y structure. For this section, to simplify notation, we consider a symmetric Y structure initially consisting of two arms, each of base pairs of dsdna, and a trunk of base pairs of dsdna (Fig. S7). As the trunk is unzipped by base pairs, nucleotides of ssdna are added to each arm while the trunk reduces to base pairs of dsdna. For simplicity, we assume that the trunk is S30
31 unzipped by two arm forces symmetric about the trunk, and the Y structure lies in the plane with the trunk end at, arm 1 end at, and arm 2 end at. The junction location and forces in each branch are determined by requiring the net force at the junction to be zero, using the measured force-extension relations. The force-extension relation per nucleotide of ssdna 15 is:, (1) where is the force, the extension per nucleotide, the contour length per nucleotide of ssdna, the persistence length of ssdna, the stretch modulus of ssdna, and the thermal energy. Using the Y structure as described in the main text (Fig. S5b), we measured,,, and to be 0.55 nm, 0.79 nm, 470 pn, and 4.11 pn nm respectively under our experimental conditions (Fig. S8). The force-extension per base pair of dsdna 10 is:, (2) where is the force, the extension per base pair, the contour length per base pair of dsdna, the persistence length of dsdna, and the stretch modulus of dsdna. We used previously measured values of,, and of 0.34 nm, 43 nm, and 1200 pn respectively 10 (Fig. S8). S31
32 Once,, and are specified and the ssdna and dsdna elastic properties are determined, the junction location is determined based on force balance, yielding the trunk extension, ssdna extension in each arm, and dsdna extension in each arm, as well as forces in each branch (Fig. S7). Thus the state of the Y structure is fully defined by the positions of the three end points of the Y structure and the number of base pairs unzipped, i.e.,, and. Below we consider unzipping under four different scenarios. The Y structure under constant end positions Consider a scenario where the trunk DNA of the Y structure is unzipped such that the ends of the arms are at specified positions (i.e., and are given and held fixed). Under thermal agitation, the fork junction may still fluctuate over multiple states, each with a different number of base pairs ( ) unzipped. We wish to find the equilibrium fork junction position and the equilibrium forces in the three branches. Our general strategy is to determine the free energies at all possible states, use these energies to define the partition function of the system, and then use the partition function to determine the equilibrium value (mean value) of any parameter of interest. The free energy of the Y structure at a given state consists of two distinct components:. (3) The first term is free energy increase due to the loss of base pairing of the first base pairs unzipped, and can be determined using the nearest-neighbor model with corrections that S32
33 take into account the temperature and salt conditions used in the experiments 26. The second term is the work to stretch each branch of the Y structure to the specified state:, (4) For ssdna of nucleotides to stretch to extension (Fig. S9), the free energy is. (5) For dsdna of base pairs to stretch to extension (Fig. S9), the free energy is. (6) Given and, we numerically calculate the extensions of all DNA segments for each possible value of using the fsolve routine of the SciPy package of Python. The results of this calculation are then used to calculate, which yields the partition function. The average number of base pairs unzipped and the average force ( = 1 to 3, one for each branch) are determined from the partition function: (7) (8) Fig. S10 shows some results of these calculations. At a small value of, the mean unzipping force along arms is comparable to the corresponding 1D unzipping force which S33
34 fluctuates around ~ 15 pn. The force along arms and the trunk force increase with a more extended Y structure in and/or more base pairs unzipped. The Y structure under constant trunk force Next we consider a scenario where the trunk DNA of the Y structure is unzipped under a constant trunk force ( ) such that the coordinates of the ends of the arms are specified (i.e., and are given and held fixed). To calculate arm force in this situation, the free energy must be a function of and instead of and. We refer to this free energy as which relates to via the Legendre transform by subtracting the product of the conjugate variables and :. (9) Since, (10) Eq. (10) allows Eq. (9) to be expressed solely in terms of,, and. Eq. (9) indicates that the free energy lowers with an increase in the trunk force. Once is determined, the arm force and the number of base pairs unzipped can be found using a partition function of the form in Eqs. (7) and (8) by replacing with. Fig. S11 shows some results of these calculations. As with the scenario where the ends of the Y structure are held at fixed positions, this scenario also shows that at low trunk S34
35 force, the arm force is comparable to the 1D unzipping force. As the trunk force increases, the arm force also increases. The Y structure under torsional constraint We now consider a scenario where the trunk end is torsionally constrained and the trunk DNA is unzipped under a constant trunk force ( ) such that the coordinates of the ends of the arms are specified (i.e., and are given and held fixed). As the trunk DNA is unzipped, the linking number in the trunk remains constant while the trunk shortens, resulting in overtwisting of the trunk. Continued torque buildup will eventually lead to a phase transition to plectonemic DNA or P-DNA We will limit our discussion to consider only the B-DNA regime prior to any phase transition. To calculate the arm force, the free energy outlined in the previous section needs to be modified to take into account the torsional energy in the trunk. Torsional energy may be expressed in terms of the degree of supercoiling,, defined as the number of turns introduced into the DNA per natural number of turns in the DNA. Under moderate forces and small degrees of supercoiling, the twist energy depends on quadratically and is also a function of the force ( ) in the DNA 31,33 :, (11) with S35
36 , (12) where is the conversion between natural angle of rotation and contour length, 100 nm the intrinsic twist persistence length 31, and contour length of the dsdna. This expression is valid until the onset of a phase transition from B-DNA to another phase. The force dependence of also implies that twist influences DNA extension. The forceextension relation of the dsdna shown in Eq. (2) needs to be slightly revised 31,33 to consider contribution from twist. For the torsionally constrained trunk in the Y structure, and are directly coupled via the number of base pairs unzipped :, and. Therefore,. (13) The free energy of the Y structure after taking into consideration the torsion in the trunk is:. (14) This additional torsional energy term is very significant as it predicts a steep increase in torsional energy even when a small number of base pairs are unzipped. In addition, to remain in the region of B-DNA, must be small (< 0.04 at 2 pn of trunk force) after which the existence of plectonemes must be considered 29. This puts a limit on the number of base pairs that can be unzipped before plectonemes begin to form in the trunk DNA. For a 4 kb trunk, this is only about ~ 160 bp. S36
37 Once is determined, the arm force and the number of base pairs unzipped can be found using a partition function of the form in Eqs. (7) and (8) by replacing with. As shown in Fig. S12, the force required to unzip torsionally constrained trunk DNA significantly differs from that for unzipping torsionally relaxed trunk DNA (Fig. S11). The steep force rise is a strong signature of torsional constraint and is readily identifiable in single molecule experiments. The Y structure under constant forces To gain an intuitive understanding of the unzipping force for the Y structure, we consider unzipping of a homopolymeric Y structure under constant forces in all three branches. For simplicity, we consider a symmetric Y structure initially consisting of no dsdna arms (i.e., ) and a trunk of base pairs of dsdna (Fig. S13). The force in each arm is held constant with a magnitude at an angle with respect to the axis. The trunk force is thus also held constant with a magnitude. For a homopolymeric DNA trunk, each base has the same magnitude of base pairing energy ( ). The free energy of the system is thus composed of the free energy increase due to the loss of base pairing of the first base pairs unzipped and the DNA stretching energy in all three branches under constant forces:. (15) We will eliminate the term that does not depend on because this term does not contribute to partitioning of the states: S37
38 . (16) Therefore, the presence of a trunk force term (the last term) which has the same sign as the base-pairing energy indicates stabilization of the trunk relative to the 1D unzipping case. When, this corresponds to 1D unzipping which has been shown to have a critical transition from DNA being fully base paired to fully unzipped as force in increased above a critical value 34,35. Therefore, we expect a similar transition to occur for unzipping of the Y structure. Indeed, since is proportional to, the minimum free energy state corresponds to either (trunk DNA remains fully double stranded) when or (trunk is fully unzipped) when. At the critical force, is independent of and thus the fork fluctuates between these extremes. As shown in Fig. S14, the calculation is valid for trunk forces < 65 pn, at which the trunk undergoes a B-S transition 36,37. As increases towards (1D unzipping limit), decreases while its z component decreases more steeply. Consequently, increases with an increase in., the x component, is greater than the 1D unzipping force over the valid range of the theory. We will specifically evaluate how and vary with as decrease from (1D unzipping). The first derivative of with respect to is: S38
39 . (17) This gives. The second derivative of with respect to at is:. (18) Since is always positive, must increase as force is applied to the trunk. Next we examine the component of the force. (19) (20) Since because dsdna has a low stiffness under a small force,. Therefore, will also increase as the trunk is extended. S39
40 References 1) Schafer, D. A., Gelles, J., Sheetz, M. P. & Landick, R. Transcription by single molecules of RNA polymerase observed by light microscopy. Nature 352, (1991). 2) Omelyanenko, V. G., Jiskoot, W. & Herron, J. N. Role of electrostatic interactions in the binding of fluorescein by anti-fluorescein antibody Biochemistry 32, (1993). 3) Bryant, Z., Stone, M. D., Gore, J., Smith, S. B., Cozzarelli, N. R. & Bustamante, C. Structural transitions and elasticity from torque measurements on DNA. Nature 424, (2003). 4) Yin, H., Wang, M. D., Svoboda, K., Landick, R., Block, S. M. & Gelles, J. Transcription against an applied force. Science 270, (1995). 5) Wang, H., Tessmer, I., Croteau, D. L., Erie, D. A. & Van Houten, B. Functional characterization and atomic force microscopy of a DNA repair protein conjugated to a quantum dot. Nano Lett 8, (2008). 6) Bai, L., Fulbright, R. M. & Wang, M. D. Mechanochemical kinetics of transcription elongation. Phys Rev Lett 98, (2007). 7) Jin, J., Bai, L., Johnson, D. S., Fulbright, R. M., Kireeva, M. L., Kashlev, M. & Wang, M. D. Synergistic action of RNA polymerases in overcoming the nucleosomal barrier. Nat Struct Mol Biol 17, (2010). 8) Moffitt, J. R., Chemla, Y. R., Izhaky, D. & Bustamante, C. Differential detection of dual traps improves the spatial resolution of optical tweezers. Proc Natl Acad Sci U S A 103, (2006). 9) Mangeol, P. & Bockelmann, U. Interference and crosstalk in double optical tweezers using a single laser source. Rev Sci Instrum 79 (2008). 10) Wang, M. D., Yin, H., Landick, R., Gelles, J. & Block, S. M. Stretching DNA with optical tweezers. Biophys J 72, (1997). 11) Koch, S. J., Shundrovsky, A., Jantzen, B. C. & Wang, M. D. Probing protein-dna interactions by unzipping a single DNA double helix. Biophys J 83, (2002). 12) Deufel, C. & Wang, M. D. Detection of forces and displacements along the axial direction in an optical trap. Biophys J 90, (2006). 13) Brower-Toland, B. & Wang, M. D. Use of optical trapping techniques to study single-nucleosome dynamics. Methods Enzymol 376, (2004). 14) Li, M. & Wang, M. D. Unzipping single DNA molecules to study nucleosome structure and dynamics. Methods Enzymol 513, (2012). S40
41 15) Smith, S. B., Cui, Y. & Bustamante, C. Overstretching B-DNA: the elastic response of individual double-stranded and single-stranded DNA molecules. Science 271, (1996). 16) Shundrovsky, A., Smith, C. L., Lis, J. T., Peterson, C. L. & Wang, M. D. Probing SWI/SNF remodeling of the nucleosome by unzipping single DNA molecules. Nat Struct Mol Biol 13, (2006). 17) Hall, M. A., Shundrovsky, A., Bai, L., Fulbright, R. M., Lis, J. T. & Wang, M. D. High-resolution dynamic mapping of histone-dna interactions in a nucleosome. Nat Struct Mol Biol 16, (2009). 18) Hegner, M., Smith, S. B. & Bustamante, C. Polymerization and mechanical properties of single RecA-DNA filaments. Proc Natl Acad Sci U S A 96, (1999). 19) Candelli, A., Hoekstra, T. P., Farge, G., Gross, P., Peterman, E. J. & Wuite, G. J. A toolbox for generating single-stranded DNA in optical tweezers experiments. Biopolymers 99, (2013). 20) Ibarra, B., Chemla, Y. R., Plyasunov, S., Smith, S. B., Lazaro, J. M., Salas, M. & Bustamante, C. Proofreading dynamics of a processive DNA polymerase. EMBO J 28, (2009). 21) Bockelmann, U., EssevazRoulet, B. & Heslot, F. Molecular stick-slip motion revealed by opening DNA with piconewton forces. Phys Rev Lett 79, (1997). 22) Dessinges, M. N., Maier, B., Zhang, Y., Peliti, M., Bensimon, D. & Croquette, V. Stretching single stranded DNA, a model polyelectrolyte. Phys Rev Lett 89, (2002). 23) Johnson, D. S., Bai, L., Smith, B. Y., Patel, S. S. & Wang, M. D. Single-molecule studies reveal dynamics of DNA unwinding by the ring-shaped T7 helicase. Cell 129, (2007). 24) Bockelmann, U., Essevaz-Roulet, B. & Heslot, F. DNA strand separation studied by single molecule force measurements. Phys Rev E 58, (1998). 25) Bockelmann, U., Thomen, P., Essevaz-Roulet, B., Viasnoff, V. & Heslot, F. Unzipping DNA with optical tweezers: high sequence sensitivity and force flips. Biophys J 82, (2002). 26) Huguet, J. M., Bizarro, C. V., Forns, N., Smith, S. B., Bustamante, C. & Ritort, F. Single-molecule derivation of salt dependent base-pair free energies in DNA. Proc Natl Acad Sci U S A 107, (2010). 27) Gross, P., Laurens, N., Oddershede, L. B., Bockelmann, U., Peterman, E. J. G. & Wuite, G. J. L. Quantifying how DNA stretches, melts and changes twist under tension. Nat Phys 7, (2011). S41
42 28) Deufel, C., Forth, S., Simmons, C. R., Dejgosha, S. & Wang, M. D. Nanofabricated quartz cylinders for angular trapping: DNA supercoiling torque detection. Nat Methods 4, (2007). 29) Forth, S., Deufel, C., Sheinin, M. Y., Daniels, B., Sethna, J. P. & Wang, M. D. Abrupt buckling transition observed during the plectoneme formation of individual DNA molecules. Phys Rev Lett 100, (2008). 30) Daniels, B. C., Forth, S., Sheinin, M. Y., Wang, M. D. & Sethna, J. P. Discontinuities at the DNA supercoiling transition. Phys Rev E Stat Nonlin Soft Matter Phys 80, (2009). 31) Sheinin, M. Y. & Wang, M. D. Twist-stretch coupling and phase transition during DNA supercoiling. Phys Chem Chem Phys 11, (2009). 32) Forth, S., Sheinin, M. Y., Inman, J. & Wang, M. D. Torque measurement at the single-molecule level. Annu Rev Biophys 42, (2013). 33) Marko, J. F. Torque and dynamics of linking number relaxation in stretched supercoiled DNA. Phys Rev E Stat Nonlin Soft Matter Phys 76, (2007). 34) Lubensky, D. K. & Nelson, D. R. Single molecule statistics and the polynucleotide unzipping transition. Phys Rev E Stat Nonlin Soft Matter Phys 65, (2002). 35) Danilowicz, C., Coljee, V. W., Bouzigues, C., Lubensky, D. K., Nelson, D. R. & Prentiss, M. DNA unzipped under a constant force exhibits multiple metastable intermediates. Proc Natl Acad Sci U S A 100, (2003). 36) King, G. A., Gross, P., Bockelmann, U., Modesti, M., Wuite, G. J. & Peterman, E. J. Revealing the competition between peeled ssdna, melting bubbles, and S-DNA during DNA overstretching using fluorescence microscopy. Proc Natl Acad Sci U S A 110, (2013). 37) Zhang, X., Chen, H., Le, S., Rouzina, I., Doyle, P. S. & Yan, J. Revealing the competition between peeled ssdna, melting bubbles, and S-DNA during DNA overstretching by single-molecule calorimetry. Proc Natl Acad Sci U S A 110, (2013). S42
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Dissecting binding of a β-barrel outer membrane
Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC
 Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Supplementary Appendixes Supplement 1: Sequences of Capture Probes. Capture probes were /5AmMC6/CTG TAG GTG CGG GTG GAC GTA GTC ACG TAG CTC CGG CTG GA-3 for vimentin, /5AmMC6/TCC CTC GCG CGT GGC TTC CGC
Supplementary Information
 Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
Supplementary Information A general solution for opening double-stranded DNA for isothermal amplification Gangyi Chen, Juan Dong, Yi Yuan, Na Li, Xin Huang, Xin Cui* and Zhuo Tang* Supplementary Materials
Supplemental Data Supplemental Figure 1.
 Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Supplemental Data Supplemental Figure 1. Silique arrangement in the wild-type, jhs, and complemented lines. Wild-type (WT) (A), the jhs1 mutant (B,C), and the jhs1 mutant complemented with JHS1 (Com) (D)
Masayoshi Honda, Jeehae Park, Robert A. Pugh, Taekjip Ha, and Maria Spies
 Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Amplified Analysis of DNA by the Autonomous Assembly of Polymers Consisting of DNAzyme Wires
 Supporting information for the paper: Amplified Analysis of DNA by the Autonomous Assembly of Polymers Consisting of DNAzyme Wires Fuan Wang, Johann Elbaz, Ron Orbach, Nimrod Magen and Itamar Willner*
Supporting information for the paper: Amplified Analysis of DNA by the Autonomous Assembly of Polymers Consisting of DNAzyme Wires Fuan Wang, Johann Elbaz, Ron Orbach, Nimrod Magen and Itamar Willner*
Supporting Online Information
 Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
Supporting Online Information Isolation of Human Genomic DNA Sequences with Expanded Nucleobase Selectivity Preeti Rathi, Sara Maurer, Grzegorz Kubik and Daniel Summerer* Department of Chemistry and Chemical
Disease and selection in the human genome 3
 Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
Disease and selection in the human genome 3 Ka/Ks revisited Please sit in row K or forward RBFD: human populations, adaptation and immunity Neandertal Museum, Mettman Germany Sequence genome Measure expression
Sequence Design for DNA Computing
 Sequence Design for DNA Computing 2004. 10. 16 Advanced AI Soo-Yong Shin and Byoung-Tak Zhang Biointelligence Laboratory DNA Hydrogen bonds Hybridization Watson-Crick Complement A single-stranded DNA molecule
Sequence Design for DNA Computing 2004. 10. 16 Advanced AI Soo-Yong Shin and Byoung-Tak Zhang Biointelligence Laboratory DNA Hydrogen bonds Hybridization Watson-Crick Complement A single-stranded DNA molecule
Probing Protein-DNA Interactions by Unzipping a Single DNA Double Helix
 1098 Biophysical Journal Volume 83 August 2002 1098 1105 Probing Protein-DNA Interactions by Unzipping a Single DNA Double Helix Steven J. Koch, Alla Shundrovsky, Benjamin C. Jantzen, and Michelle D. Wang
1098 Biophysical Journal Volume 83 August 2002 1098 1105 Probing Protein-DNA Interactions by Unzipping a Single DNA Double Helix Steven J. Koch, Alla Shundrovsky, Benjamin C. Jantzen, and Michelle D. Wang
Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis
 1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
1 2 3 4 5 6 7 8 9 10 11 12 Figure S1. Characterization of the irx9l-1 mutant. (A) Diagram of the Arabidopsis IRX9L gene drawn based on information from TAIR (the Arabidopsis Information Research). Exons
Wet Lab Tutorial: Genelet Circuits
 Wet Lab Tutorial: Genelet Circuits DNA 17 This tutorial will introduce the in vitro transcriptional circuits toolkit. The tutorial will focus on the design, synthesis, and experimental testing of a synthetic
Wet Lab Tutorial: Genelet Circuits DNA 17 This tutorial will introduce the in vitro transcriptional circuits toolkit. The tutorial will focus on the design, synthesis, and experimental testing of a synthetic
Supplementary Information. Construction of Lasso Peptide Fusion Proteins
 Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
Supplementary Information Construction of Lasso Peptide Fusion Proteins Chuhan Zong 1, Mikhail O. Maksimov 2, A. James Link 2,3 * Departments of 1 Chemistry, 2 Chemical and Biological Engineering, and
strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular
![strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular strain devoid of the aox1 gene [1]. Thus, the identification of AOX1 in the intracellular](/thumbs/72/66361212.jpg) Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Additional file 2 Identification of AOX1 in P. pastoris GS115 with a Mut s phenotype Results and Discussion The HBsAg producing strain was originally identified as a Mut s (methanol utilization slow) strain
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for Expanding the Genetic
Genomics and Gene Recognition Genes and Blue Genes
 Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
Genomics and Gene Recognition Genes and Blue Genes November 1, 2004 Prokaryotic Gene Structure prokaryotes are simplest free-living organisms studying prokaryotes can give us a sense what is the minimum
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Amplified Binding-Induced Homogeneous Assay through Catalytic
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Amplified Binding-Induced Homogeneous Assay through Catalytic
SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer
 TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
TEACHER S GUIDE SAY IT WITH DNA: Protein Synthesis Activity by Larry Flammer SYNOPSIS This activity uses the metaphor of decoding a secret message for the Protein Synthesis process. Students teach themselves
Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH).
 Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
Bisulfite Treatment of DNA Dilute DNA sample to 2µg DNA in 50µl ddh 2 O. Add 5µl of 3N NaOH to DNA sample (final concentration 0.3N NaOH). Incubate in a 37ºC water bath for 30 minutes. To 55µl samples
[5] Use of Optical Trapping Techniques to Study Single-Nucleosome Dynamics
![[5] Use of Optical Trapping Techniques to Study Single-Nucleosome Dynamics [5] Use of Optical Trapping Techniques to Study Single-Nucleosome Dynamics](/thumbs/72/67186550.jpg) 62 chromatin proteins [5] of the RSC complex from the unstained particle images entails combining the information present in this set of projections. This requires precise knowledge of their relative orientations.
62 chromatin proteins [5] of the RSC complex from the unstained particle images entails combining the information present in this set of projections. This requires precise knowledge of their relative orientations.
Replication. Obaidur Rahman
 Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
Supplemental Data. mir156-regulated SPL Transcription. Factors Define an Endogenous Flowering. Pathway in Arabidopsis thaliana
 Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
Cell, Volume 138 Supplemental Data mir156-regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana Jia-Wei Wang, Benjamin Czech, and Detlef Weigel Table S1. Interaction
G+C content. 1 Introduction. 2 Chromosomes Topology & Counts. 3 Genome size. 4 Replichores and gene orientation. 5 Chirochores.
 1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
1 Introduction 2 Chromosomes Topology & Counts 3 Genome size 4 Replichores and gene orientation 5 Chirochores 6 7 Codon usage 121 marc.bailly-bechet@univ-lyon1.fr Bacterial genome structures Introduction
Interpretation of sequence results
 Interpretation of sequence results An overview on DNA sequencing: DNA sequencing involves the determination of the sequence of nucleotides in a sample of DNA. It use a modified PCR reaction where both
Interpretation of sequence results An overview on DNA sequencing: DNA sequencing involves the determination of the sequence of nucleotides in a sample of DNA. It use a modified PCR reaction where both
Lecture Four. Molecular Approaches I: Nucleic Acids
 Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
HiPer Real-Time PCR Teaching Kit
 HiPer Real-Time PCR Teaching Kit Product Code: HTBM032 Number of experiments that can be performed: 10 Duration of Experiment Protocol: 1.5 hours Storage Instructions: The kit is stable for 12 months from
HiPer Real-Time PCR Teaching Kit Product Code: HTBM032 Number of experiments that can be performed: 10 Duration of Experiment Protocol: 1.5 hours Storage Instructions: The kit is stable for 12 months from
A green method of staining DNA in polyacrylamide gel electrophoresis based on fluorescent copper nanoclusters synthesized in situ
 Electronic Supplementary Material A green method of staining DNA in polyacrylamide gel electrophoresis based on fluorescent copper nanoclusters synthesized in situ Xiaoli Zhu 1, Hai Shi 1, Yalan Shen 1,
Electronic Supplementary Material A green method of staining DNA in polyacrylamide gel electrophoresis based on fluorescent copper nanoclusters synthesized in situ Xiaoli Zhu 1, Hai Shi 1, Yalan Shen 1,
Enzymatic assembly of DNA molecules up to several hundred kilobases
 nature methods Enzymatic assembly of DNA molecules up to several hundred kilobases Daniel G Gibson, Lei Young, Ray-Yuan Chuang, J Craig Venter, Clyde A Hutchison III & Hamilton O Smith Supplementary figures
nature methods Enzymatic assembly of DNA molecules up to several hundred kilobases Daniel G Gibson, Lei Young, Ray-Yuan Chuang, J Craig Venter, Clyde A Hutchison III & Hamilton O Smith Supplementary figures
Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR
 Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
Supplemental Dataset Supplemental Table 1. Mutant ADAMTS3 alleles detected in HEK293T clone 4C2. DNA sequence Amino acid sequence WT CCTGTCACTTTGGTTGATAGC MVLLSLWLIAAALVEVR Allele 1 CCTGTC------------------GATAGC
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
www.sciencesignaling.org/cgi/content/full/10/494/eaan6284/dc1 Supplementary Materials for Activation of master virulence regulator PhoP in acidic ph requires the Salmonella-specific protein UgtL Jeongjoon
Elecrtonic Supplementary Information. Application of quantum dot barcodes prepared using biological self-assembly to multiplexed immunoassays
 Elecrtonic Supplementary Information Application of quantum dot barcodes prepared using biological self-assembly to multiplexed immunoassays Sakandar Rauf, Andrew Glidle and Jonathan M Cooper Department
Elecrtonic Supplementary Information Application of quantum dot barcodes prepared using biological self-assembly to multiplexed immunoassays Sakandar Rauf, Andrew Glidle and Jonathan M Cooper Department
Materials and methods. by University of Washington Yeast Resource Center) from several promoters, including
 Supporting online material for Elowitz et al. report Materials and methods Strains and plasmids. Plasmids expressing CFP or YFP (wild-type codons, developed by University of Washington Yeast Resource Center)
Supporting online material for Elowitz et al. report Materials and methods Strains and plasmids. Plasmids expressing CFP or YFP (wild-type codons, developed by University of Washington Yeast Resource Center)
Lecture 11: Gene Prediction
 Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
Lecture 11: Gene Prediction Study Chapter 6.11-6.14 1 Gene: A sequence of nucleotides coding for protein Gene Prediction Problem: Determine the beginning and end positions of genes in a genome Where are
Table S1. Bacterial strains (Related to Results and Experimental Procedures)
 Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
Table S1. Bacterial strains (Related to Results and Experimental Procedures) Strain number Relevant genotype Source or reference 1045 AB1157 Graham Walker (Donnelly and Walker, 1989) 2458 3084 (MG1655)
Structure of DNA [pln39]
![Structure of DNA [pln39] Structure of DNA [pln39]](/thumbs/77/76253099.jpg) Structure of DNA [pln39] Deoxyribonucleic acid (DNA) consists of two biopolymer strands cross-linked into a double helix. Each strand is a polynucleotide. Composition of nucleotide: nucleobase: guanine
Structure of DNA [pln39] Deoxyribonucleic acid (DNA) consists of two biopolymer strands cross-linked into a double helix. Each strand is a polynucleotide. Composition of nucleotide: nucleobase: guanine
Final exam. Please write your name on the exam and keep an ID card ready.
 Biophysics of Macromolecules Prof. R. Jungmann and Prof. J. Lipfert SS 2017 Final exam Final exam First name: Last name: Student number ( Matrikelnummer ): Please write your name on the exam and keep an
Biophysics of Macromolecules Prof. R. Jungmann and Prof. J. Lipfert SS 2017 Final exam Final exam First name: Last name: Student number ( Matrikelnummer ): Please write your name on the exam and keep an
Supporting Information. Trifluoroacetophenone-Linked Nucleotides and DNA for Studying of DNA-protein Interactions by 19 F NMR Spectroscopy
 Supporting Information Trifluoroacetophenone-Linked Nucleotides and DNA for Studying of DNA-protein Interactions by 19 F NMR Spectroscopy Agata Olszewska, Radek Pohl and Michal Hocek # * Institute of Organic
Supporting Information Trifluoroacetophenone-Linked Nucleotides and DNA for Studying of DNA-protein Interactions by 19 F NMR Spectroscopy Agata Olszewska, Radek Pohl and Michal Hocek # * Institute of Organic
Stretching DNA as a template for molecular construction
 title Workshop " DNA-Based Molecular Electronics" Jena, May 13-15, 2004 Stretching DNA as a template for molecular construction Masao Washizu 1, Yuji Kimura 1, Takuya Kobayashi 1, Osamu Kurosawa 1,2 Sayoko
title Workshop " DNA-Based Molecular Electronics" Jena, May 13-15, 2004 Stretching DNA as a template for molecular construction Masao Washizu 1, Yuji Kimura 1, Takuya Kobayashi 1, Osamu Kurosawa 1,2 Sayoko
Gene Expression Technology
 Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Module1TheBasicsofRealTimePCR Monday, March 19, 2007
 Objectives Slide notes: Page 1 of 41 Module 1: The Basics Of Real Time PCR Slide notes: Module 1: The Basics of real time PCR Page 2 of 41 Polymerase Chain Reaction Slide notes: Here is a review of PCR,
Objectives Slide notes: Page 1 of 41 Module 1: The Basics Of Real Time PCR Slide notes: Module 1: The Basics of real time PCR Page 2 of 41 Polymerase Chain Reaction Slide notes: Here is a review of PCR,
Structure of nucleic acids II Biochemistry 302. January 20, 2006
 Structure of nucleic acids II Biochemistry 302 January 20, 2006 Intrinsic structural flexibility of RNA antiparallel A-form Fig. 4.19 High Temp Denaturants In vivo conditions Base stacking w/o base pairing/h-bonds
Structure of nucleic acids II Biochemistry 302 January 20, 2006 Intrinsic structural flexibility of RNA antiparallel A-form Fig. 4.19 High Temp Denaturants In vivo conditions Base stacking w/o base pairing/h-bonds
Fig. 16-7a. 5 end Hydrogen bond 3 end. 1 nm. 3.4 nm nm
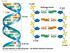 Fig. 16-7a end Hydrogen bond end 1 nm 3.4 nm 0.34 nm (a) Key features of DNA structure end (b) Partial chemical structure end Fig. 16-8 Adenine (A) Thymine (T) Guanine (G) Cytosine (C) Concept 16.2: Many
Fig. 16-7a end Hydrogen bond end 1 nm 3.4 nm 0.34 nm (a) Key features of DNA structure end (b) Partial chemical structure end Fig. 16-8 Adenine (A) Thymine (T) Guanine (G) Cytosine (C) Concept 16.2: Many
Maria Spies, Mark S. Dillingham, and Stephen C. Kowalczykowski. Sections of Microbiology, and of Molecular and Cellular Biology, Center for
 DNA HELICASES Maria Spies, Mark S. Dillingham, and Stephen C. Kowalczykowski Sections of Microbiology, and of Molecular and Cellular Biology, Center for Genetics and Development, University of California,
DNA HELICASES Maria Spies, Mark S. Dillingham, and Stephen C. Kowalczykowski Sections of Microbiology, and of Molecular and Cellular Biology, Center for Genetics and Development, University of California,
The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity
 Promega Notes Magazine Number 62, 1997, p. 02 The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity By Christine Andrews and Scott Lesley Promega
Promega Notes Magazine Number 62, 1997, p. 02 The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity By Christine Andrews and Scott Lesley Promega
Engineering D66N mutant using quick change site directed mutagenesis. Harkewal Singh 09/01/2010
 Engineering D66N mutant using quick change site directed mutagenesis Harkewal Singh 09/01/2010 1 1- What is quick change site directed mutagenesis? 2- An overview of the kit contents. 3- A brief information
Engineering D66N mutant using quick change site directed mutagenesis Harkewal Singh 09/01/2010 1 1- What is quick change site directed mutagenesis? 2- An overview of the kit contents. 3- A brief information
 The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
Supporting Information
 Supporting Information Velocity of DNA during translocation through a solid state nanopore Calin Plesa, Nick van Loo, Philip Ketterer, Hendrik Dietz, Cees Dekker Department of Bionanoscience, Kavli Institute
Supporting Information Velocity of DNA during translocation through a solid state nanopore Calin Plesa, Nick van Loo, Philip Ketterer, Hendrik Dietz, Cees Dekker Department of Bionanoscience, Kavli Institute
II 0.95 DM2 (RPP1) DM3 (At3g61540) b
 Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
Table S2. F 2 Segregation Ratios at 16 C, Related to Figure 2 Cross n c Phenotype Model e 2 Locus A Locus B Normal F 1 -like Enhanced d Uk-1/Uk-3 149 64 36 49 DM2 (RPP1) DM1 (SSI4) a Bla-1/Hh-0 F 3 111
Chapter 13 Chromatin Structure and its Effects on Transcription
 Chapter 13 Chromatin Structure and its Effects on Transcription Students must be positive that they understand standard PCR. There is a resource on the web for this purpose. Warn them before this class.
Chapter 13 Chromatin Structure and its Effects on Transcription Students must be positive that they understand standard PCR. There is a resource on the web for this purpose. Warn them before this class.
Biological Nanomachines
 Biological Nanomachines Yann R Chemla Dept. of Physics, University of Illinois at Urbana Champaign Saturday Physics for Everyone, Sept. 14, 2013 Biophysics at Illinois Part I: WHAT IS BIOPHYSICS? Physicists
Biological Nanomachines Yann R Chemla Dept. of Physics, University of Illinois at Urbana Champaign Saturday Physics for Everyone, Sept. 14, 2013 Biophysics at Illinois Part I: WHAT IS BIOPHYSICS? Physicists
Electronic Supplementary Information Sensitive detection of polynucleotide kinase using rolling circle amplification-induced chemiluminescence
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sensitive detection of polynucleotide kinase
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sensitive detection of polynucleotide kinase
QuantiFluor ONE dsdna System
 TECHNICAL MANUAL QuantiFluor ONE dsdna System Instructions for Use of Products E4871 and E4870 Revised 6/17 TM405 QuantiFluor ONE dsdna System All technical literature is available at: www.promega.com/protocols/
TECHNICAL MANUAL QuantiFluor ONE dsdna System Instructions for Use of Products E4871 and E4870 Revised 6/17 TM405 QuantiFluor ONE dsdna System All technical literature is available at: www.promega.com/protocols/
DNA Replication II Biochemistry 302. January 25, 2006
 DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
MCB 110:Biochemistry of the Central Dogma of MB. MCB 110:Biochemistry of the Central Dogma of MB
 MCB 110:Biochemistry of the Central Dogma of MB Part 1. DNA replication, repair and genomics (Prof. Alber) Part 2. RNA & protein synthesis. Prof. Zhou Part 3. Membranes, protein secretion, trafficking
MCB 110:Biochemistry of the Central Dogma of MB Part 1. DNA replication, repair and genomics (Prof. Alber) Part 2. RNA & protein synthesis. Prof. Zhou Part 3. Membranes, protein secretion, trafficking
Recombinant DNA Technology
 History of recombinant DNA technology Recombinant DNA Technology (DNA cloning) Majid Mojarrad Recombinant DNA technology is one of the recent advances in biotechnology, which was developed by two scientists
History of recombinant DNA technology Recombinant DNA Technology (DNA cloning) Majid Mojarrad Recombinant DNA technology is one of the recent advances in biotechnology, which was developed by two scientists
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Chapter 10: Classification of Microorganisms
 Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy 8 successive taxa
Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy 8 successive taxa
qpcr Quantitative PCR or Real-time PCR Gives a measurement of PCR product at end of each cycle real time
 qpcr qpcr Quantitative PCR or Real-time PCR Gives a measurement of PCR product at end of each cycle real time Differs from endpoint PCR gel on last cycle Used to determines relative amount of template
qpcr qpcr Quantitative PCR or Real-time PCR Gives a measurement of PCR product at end of each cycle real time Differs from endpoint PCR gel on last cycle Used to determines relative amount of template
Event-specific Method for the Quantification of Soybean Line A Using Real-time PCR. Protocol
 Event-specific Method for the Quantification of Soybean Line A2704-12 Using Real-time PCR Protocol 14 May 2007 Directorate General-Joint Research Centre Institute for Health and Consumer Protection Biotechnology
Event-specific Method for the Quantification of Soybean Line A2704-12 Using Real-time PCR Protocol 14 May 2007 Directorate General-Joint Research Centre Institute for Health and Consumer Protection Biotechnology
NAP1-Assisted Nucleosome Assembly on DNA Measured in Real Time by Single-Molecule Magnetic Tweezers
 NAP1-Assisted Nucleosome Assembly on DNA Measured in Real Time by Single-Molecule Magnetic Tweezers Rifka Vlijm 1, Jeremy S. J. Smitshuijzen 1, Alexandra Lusser 2, Cees Dekker 1 * 1 Department of Bionanoscience,
NAP1-Assisted Nucleosome Assembly on DNA Measured in Real Time by Single-Molecule Magnetic Tweezers Rifka Vlijm 1, Jeremy S. J. Smitshuijzen 1, Alexandra Lusser 2, Cees Dekker 1 * 1 Department of Bionanoscience,
The B1 Protein Guides the Biosynthesis of a Lasso Peptide
 The B1 Protein Guides the Biosynthesis of a Lasso Peptide Shaozhou Zhu 1,2, Christopher D. Fage 1, Julian D. Hegemann 1, Andreas Mielcarek 1, Dushan Yan 1, Uwe Linne 1 & Mohamed A. Marahiel*,1 1 Department
The B1 Protein Guides the Biosynthesis of a Lasso Peptide Shaozhou Zhu 1,2, Christopher D. Fage 1, Julian D. Hegemann 1, Andreas Mielcarek 1, Dushan Yan 1, Uwe Linne 1 & Mohamed A. Marahiel*,1 1 Department
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2014. Supporting Information for Small, DOI: 10.1002/smll.201303558 Ultraspecific and Highly Sensitive Nucleic Acid Detection by Integrating
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2014. Supporting Information for Small, DOI: 10.1002/smll.201303558 Ultraspecific and Highly Sensitive Nucleic Acid Detection by Integrating
Technical Review. Real time PCR
 Technical Review Real time PCR Normal PCR: Analyze with agarose gel Normal PCR vs Real time PCR Real-time PCR, also known as quantitative PCR (qpcr) or kinetic PCR Key feature: Used to amplify and simultaneously
Technical Review Real time PCR Normal PCR: Analyze with agarose gel Normal PCR vs Real time PCR Real-time PCR, also known as quantitative PCR (qpcr) or kinetic PCR Key feature: Used to amplify and simultaneously
Event-specific Method for the Quantification of Soybean CV127 Using Real-time PCR. Protocol
 Event-specific Method for the Quantification of Soybean CV127 Using Real-time PCR Protocol 20 September 2011 Joint Research Centre Institute for Health and Consumer Protection Molecular Biology and Genomics
Event-specific Method for the Quantification of Soybean CV127 Using Real-time PCR Protocol 20 September 2011 Joint Research Centre Institute for Health and Consumer Protection Molecular Biology and Genomics
Computational Biology I LSM5191
 Computational Biology I LSM5191 Lecture 5 Notes: Genetic manipulation & Molecular Biology techniques Broad Overview of: Enzymatic tools in Molecular Biology Gel electrophoresis Restriction mapping DNA
Computational Biology I LSM5191 Lecture 5 Notes: Genetic manipulation & Molecular Biology techniques Broad Overview of: Enzymatic tools in Molecular Biology Gel electrophoresis Restriction mapping DNA
Supplementary Fig. 1. Isolation and in vitro expansion of EpCAM + cholangiocytes. For collagenase perfusion, enzyme solution was injected from the
 Supplementary Fig. 1. Isolation and in vitro expansion of EpCAM + cholangiocytes. For collagenase perfusion, enzyme solution was injected from the portal vein for digesting adult livers, whereas it was
Supplementary Fig. 1. Isolation and in vitro expansion of EpCAM + cholangiocytes. For collagenase perfusion, enzyme solution was injected from the portal vein for digesting adult livers, whereas it was
DNA Hybridization and Detection
 Chapter 6 DNA Hybridization and Detection Fluorescence Polarization Detection of DNA Hybridization........................................................ 6-2 Introduction.............................................................................................................
Chapter 6 DNA Hybridization and Detection Fluorescence Polarization Detection of DNA Hybridization........................................................ 6-2 Introduction.............................................................................................................
A high efficient electrochemiluminescence resonance energy. transfer system in one nanostructure: its application for
 Supporting Information for A high efficient electrochemiluminescence resonance energy transfer system in one nanostructure: its application for ultrasensitive detection of microrna in cancer cells Zhaoyang
Supporting Information for A high efficient electrochemiluminescence resonance energy transfer system in one nanostructure: its application for ultrasensitive detection of microrna in cancer cells Zhaoyang
Human Granulin / GRN / Progranulin ELISA Pair Set
 Human Granulin / GRN / Progranulin ELISA Pair Set Catalog Number : SEKA10826 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
Human Granulin / GRN / Progranulin ELISA Pair Set Catalog Number : SEKA10826 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
NEBNext Multiplex Oligos for Illumina (Index Primers Set 3)
 LIBRARY PREPARATION NEBNext Multiplex Oligos for (Index Primers Set 3) Instruction Manual NEB #E7710S/L 24/96 reactions This product is intended for research purposes only. This product is not intended
LIBRARY PREPARATION NEBNext Multiplex Oligos for (Index Primers Set 3) Instruction Manual NEB #E7710S/L 24/96 reactions This product is intended for research purposes only. This product is not intended
Polymerase Chain Reaction (PCR)
 Laboratory for Environmental Pathogens Research Department of Environmental Sciences University of Toledo Polymerase Chain Reaction (PCR) Background information The polymerase chain reaction (PCR) is an
Laboratory for Environmental Pathogens Research Department of Environmental Sciences University of Toledo Polymerase Chain Reaction (PCR) Background information The polymerase chain reaction (PCR) is an
XactEdit Cas9 Nuclease with NLS User Manual
 XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
Chapter 3. Enzyme manipulation of DNA and RNA
 Chapter 3 Enzyme manipulation of DNA and RNA To measure incorporation of radioactivity (to see if the probe is good or not for hybridization) Acid precipitation method: - Add sonicated salmon sperm DNA
Chapter 3 Enzyme manipulation of DNA and RNA To measure incorporation of radioactivity (to see if the probe is good or not for hybridization) Acid precipitation method: - Add sonicated salmon sperm DNA
Structure of nucleic acids II Biochemistry 302. Bob Kelm January 21, 2005
 Structure of nucleic acids II Biochemistry 302 Bob Kelm January 21, 2005 http://biochem.uvm.edu/courses/kelm/302 User: student PW: nucleicacid Secondary structure of RNAs antiparallel A-form Fig. 4.19
Structure of nucleic acids II Biochemistry 302 Bob Kelm January 21, 2005 http://biochem.uvm.edu/courses/kelm/302 User: student PW: nucleicacid Secondary structure of RNAs antiparallel A-form Fig. 4.19
Cat # Box1 Box2. DH5a Competent E. coli cells CCK-20 (20 rxns) 40 µl 40 µl 50 µl x 20 tubes. Choo-Choo Cloning TM Enzyme Mix
 Molecular Cloning Laboratories User Manual Version 3.3 Product name: Choo-Choo Cloning Kits Cat #: CCK-10, CCK-20, CCK-096, CCK-384 Description: Choo-Choo Cloning is a highly efficient directional PCR
Molecular Cloning Laboratories User Manual Version 3.3 Product name: Choo-Choo Cloning Kits Cat #: CCK-10, CCK-20, CCK-096, CCK-384 Description: Choo-Choo Cloning is a highly efficient directional PCR
NEBNext Multiplex Oligos for Illumina (Index Primers Set 4)
 LIBRARY PREPARATION NEBNext Multiplex Oligos for (Index Primers Set 4) Instruction Manual NEB #E7730S/L 24/96 reactions Version 2.0 12/16 be INSPIRED drive DISCOVERY stay GENUINE This product is intended
LIBRARY PREPARATION NEBNext Multiplex Oligos for (Index Primers Set 4) Instruction Manual NEB #E7730S/L 24/96 reactions Version 2.0 12/16 be INSPIRED drive DISCOVERY stay GENUINE This product is intended
MCB421 FALL2005 EXAM#3 ANSWERS Page 1 of 12. ANSWER: Both transposon types form small duplications of adjacent host DNA sequences.
 Page 1 of 12 (10pts) 1. There are two mechanisms for transposition used by bacterial transposable elements: replicative (Tn3) and non-replicative (Tn5 and Tn10). Compare and contrast the two mechanisms
Page 1 of 12 (10pts) 1. There are two mechanisms for transposition used by bacterial transposable elements: replicative (Tn3) and non-replicative (Tn5 and Tn10). Compare and contrast the two mechanisms
Roche Molecular Biochemicals Technical Note No. LC 10/2000
 Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
1. I can describe the stages of the cell cycle.
 Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Genetics and Genomics in Medicine Chapter 3. Questions & Answers
 Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Supporting Information
 Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Site-Specific Control of Distances between Gold Nanoparticles using Phosphorothioate Anchors on DNA and a Short Bifunctional Molecular Fastener
Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Site-Specific Control of Distances between Gold Nanoparticles using Phosphorothioate Anchors on DNA and a Short Bifunctional Molecular Fastener
Circular bivalent aptamers enable in vivo stability and recognition
 Supporting Information Circular bivalent aptamers enable in vivo stability and recognition Hailan Kuai,, Zilong Zhao,, Liuting Mo, Hui Liu, Xiaoxiao Hu, Ting Fu, Xiaobing Zhang, Weihong Tan*,, Molecular
Supporting Information Circular bivalent aptamers enable in vivo stability and recognition Hailan Kuai,, Zilong Zhao,, Liuting Mo, Hui Liu, Xiaoxiao Hu, Ting Fu, Xiaobing Zhang, Weihong Tan*,, Molecular
Stellaris RNA FISH Protocol for Simultaneous IF + FISH in Adherent Cells
 Stellaris RNA FISH Protocol for Simultaneous IF + FISH in Adherent Cells General Protocol & Storage Product Description A set of Stellaris RNA FISH Probes is comprised of up to 48 singly labeled oligonucleotides
Stellaris RNA FISH Protocol for Simultaneous IF + FISH in Adherent Cells General Protocol & Storage Product Description A set of Stellaris RNA FISH Probes is comprised of up to 48 singly labeled oligonucleotides
Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers
 DNA Research 9, 163 171 (2002) Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers Shinobu Nasu, Junko Suzuki, Rieko
DNA Research 9, 163 171 (2002) Search for and Analysis of Single Nucleotide Polymorphisms (SNPs) in Rice (Oryza sativa, Oryza rufipogon) and Establishment of SNP Markers Shinobu Nasu, Junko Suzuki, Rieko
Universal Split Spinach Aptamer (USSA) for Nucleic Acid Analysis and DNA Computation
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting materials Universal Split Spinach Aptamer (USSA) for Nucleic Acid Analysis and DNA Computation
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting materials Universal Split Spinach Aptamer (USSA) for Nucleic Acid Analysis and DNA Computation
Proposed Models of DNA Replication. Conservative Model. Semi-Conservative Model. Dispersive model
 5.2 DNA Replication Cell Cycle Life cycle of a cell Cells can reproduce Daughter cells receive an exact copy of DNA from parent cell DNA replication happens during the S phase Proposed Models of DNA Replication
5.2 DNA Replication Cell Cycle Life cycle of a cell Cells can reproduce Daughter cells receive an exact copy of DNA from parent cell DNA replication happens during the S phase Proposed Models of DNA Replication
UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change
 A p p l i c a t i o n N o t e UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change Using Intrinsic Tryptophan Fluorescence in Conjunction with UV-capable Polarizers
A p p l i c a t i o n N o t e UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change Using Intrinsic Tryptophan Fluorescence in Conjunction with UV-capable Polarizers
Session 3 Cloning Overview & Polymerase Chain Reaction
 Session 3 Cloning Overview & Polymerase Chain Reaction Learning Objective: In this lab exercise, you will become familiar with the steps of a polymerase chain reaction, the required reagents for a successful
Session 3 Cloning Overview & Polymerase Chain Reaction Learning Objective: In this lab exercise, you will become familiar with the steps of a polymerase chain reaction, the required reagents for a successful
PROTEIN SYNTHESIS Study Guide
 PART A. Read the following: PROTEIN SYNTHESIS Study Guide Protein synthesis is the process used by the body to make proteins. The first step of protein synthesis is called Transcription. It occurs in the
PART A. Read the following: PROTEIN SYNTHESIS Study Guide Protein synthesis is the process used by the body to make proteins. The first step of protein synthesis is called Transcription. It occurs in the
Quantitative Real time PCR. Only for teaching purposes - not for reproduction or sale
 Quantitative Real time PCR PCR reaction conventional versus real time PCR real time PCR principles threshold cycle C T efficiency relative quantification reference genes primers detection chemistry GLP
Quantitative Real time PCR PCR reaction conventional versus real time PCR real time PCR principles threshold cycle C T efficiency relative quantification reference genes primers detection chemistry GLP
Supplemental Information. Target-Mediated Protection of Endogenous. MicroRNAs in C. elegans. Inventory of Supplementary Information
 Developmental Cell, Volume 20 Supplemental Information Target-Mediated Protection of Endogenous MicroRNAs in C. elegans Saibal Chatterjee, Monika Fasler, Ingo Büssing, and Helge Großhans Inventory of Supplementary
Developmental Cell, Volume 20 Supplemental Information Target-Mediated Protection of Endogenous MicroRNAs in C. elegans Saibal Chatterjee, Monika Fasler, Ingo Büssing, and Helge Großhans Inventory of Supplementary
Chapter 17. PCR the polymerase chain reaction and its many uses. Prepared by Woojoo Choi
 Chapter 17. PCR the polymerase chain reaction and its many uses Prepared by Woojoo Choi Polymerase chain reaction 1) Polymerase chain reaction (PCR): artificial amplification of a DNA sequence by repeated
Chapter 17. PCR the polymerase chain reaction and its many uses Prepared by Woojoo Choi Polymerase chain reaction 1) Polymerase chain reaction (PCR): artificial amplification of a DNA sequence by repeated
Protocol T45 Community Reference Laboratory for GM Food and Feed
 EUROPEAN COMMISSION DIRECTORATE GENERAL JRC JOINT RESEARCH CENTRE INSTITUTE FOR HEALTH AND CONSUMER PROTECTION COMMUNITY REFERENCE LABORATORY FOR GM FOOD AND FEED Event-specific Method for the Quantification
EUROPEAN COMMISSION DIRECTORATE GENERAL JRC JOINT RESEARCH CENTRE INSTITUTE FOR HEALTH AND CONSUMER PROTECTION COMMUNITY REFERENCE LABORATORY FOR GM FOOD AND FEED Event-specific Method for the Quantification
Thermo Scientific GENESYS 10S Bio spectrophotometer
 PRODUCT SPECIFICATIONS GENESYS 10S Bio UV-Visible Spectrophotometer Thermo Scientific GENESYS 10S Bio spectrophotometer Accurate and convenient life science UV-Visible measurements Designed for performance
PRODUCT SPECIFICATIONS GENESYS 10S Bio UV-Visible Spectrophotometer Thermo Scientific GENESYS 10S Bio spectrophotometer Accurate and convenient life science UV-Visible measurements Designed for performance
Coexistence of Twisted, Plectonemic, and Melted DNA in Small Topological Domains
 1174 Biophysical Journal Volume 106 March 2014 1174 1181 Coexistence of Twisted, Plectonemic, and Melted DNA in Small Topological Domains He Meng, Johan Bosman, Thijn van der Heijden, and John van Noort*
1174 Biophysical Journal Volume 106 March 2014 1174 1181 Coexistence of Twisted, Plectonemic, and Melted DNA in Small Topological Domains He Meng, Johan Bosman, Thijn van der Heijden, and John van Noort*
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Replication Review. 1. What is DNA Replication? 2. Where does DNA Replication take place in eukaryotic cells?
 Replication Review 1. What is DNA Replication? 2. Where does DNA Replication take place in eukaryotic cells? 3. Where does DNA Replication take place in the cell cycle? 4. 4. What guides DNA Replication?
Replication Review 1. What is DNA Replication? 2. Where does DNA Replication take place in eukaryotic cells? 3. Where does DNA Replication take place in the cell cycle? 4. 4. What guides DNA Replication?
Evaluation of Mechanical Properties of Hard Coatings
 Evaluation of Mechanical Properties of Hard Coatings Comprehensive mechanical testing of two coated metal samples was performed on the UNMT- 1. The tests clearly distinguished brittle and ductile samples,
Evaluation of Mechanical Properties of Hard Coatings Comprehensive mechanical testing of two coated metal samples was performed on the UNMT- 1. The tests clearly distinguished brittle and ductile samples,
12 2 Chromosomes and DNA Replication
 DNA Replication 12-2 Chromosomes and 1 of 21 DNA and Chromosomes DNA and Chromosomes In prokaryotic cells, DNA is located in the cytoplasm. Most prokaryotes have a single DNA molecule containing nearly
DNA Replication 12-2 Chromosomes and 1 of 21 DNA and Chromosomes DNA and Chromosomes In prokaryotic cells, DNA is located in the cytoplasm. Most prokaryotes have a single DNA molecule containing nearly
DNA Replication. The Organization of DNA. Recall:
 Recall: The Organization of DNA DNA Replication Chromosomal form appears only during mitosis, and is used in karyotypes. folded back upon itself (chromosomes) coiled around itself (chromatin) wrapped around
Recall: The Organization of DNA DNA Replication Chromosomal form appears only during mitosis, and is used in karyotypes. folded back upon itself (chromosomes) coiled around itself (chromatin) wrapped around
