Supplementary Information
|
|
|
- Sydney Lindsey
- 5 years ago
- Views:
Transcription
1 Supplementary Information This Supplementary Information file contains the following contents: Supplementary Methods, Supplementary Discussion, Supplementary Figures (S1~S9), Supplementary Tables (1~3) and Supplementary Sequences. Supplementary Methods Animals The rat used in this study was the Specific pathogen-free (SPF) grade Sprague-Dawley (SD) strain. Rats were purchased from Beijing Vital River laboratory animal center. All animal experiments were performed in compliance with the guidelines of the Institute of Zoology, Chinese Academy of Sciences. Cas9 and sgrna vector construct The Cas9 sequence was consistent with previous report, and was ordered from Taihe Biotechnology Company (Beijing), then cloned into the peasy-t1 vector (Transgen). The sgrna design was similar as Hwang et al. described, with difference that the 5 (GG) was not required in our study 1. sgrnas together were also ordered from Taihe Biotechnology Company (Beijing). The vector sequences containing the backbone and Cas9 or sgrna were shown in the supplementary sequences. In vitro preparation of sgrnas and Cas9 mrna for microinjection RNAs needed for microinjection were in vitro transcribed. sgrnas and Cas9 mrna were transcribed by the HiScribe T7 In Vitro Transcription Kit (NEB) according to manufacturer s instructions. During in vitro transcription the Cas9 mrna was capped with the m7g(5')ppp(5')g RNA Cap Structure Analog (NEB) by T7 transcriptase. RNAs were dissolved in DEPC water and stored in -80. Intracytoplasmic RNA microinjection The intracytoplasmic RNA microinjection was performed according to previous report 2. Briefly, one-cell-stage embryos were collected at 0.5 day post coitum (dpc). Each embryo was microinjected with 10 ng/μl sgrna and 60 ng/μl Cas9 mrna into the cytoplasm. For multiple gene targeting, each sgrna was at 10 ng/μl concentration and the Cas9 mrna was still at 60 ng/μl. After microinjection, surviving embryos were implanted on the same day in the oviduct of pseudopregnant SD female rats. Full-term pups were obtained by natural labour at 21.5 dpc. Surveyor nuclease assay and indel rate analysis The Surveyor nuclease assay for detection of genetic mutation was performed with the SURVEYOR Mutation Detection Kit for Standard Gel Electrophoresis (Transgenomics) according to the manufacturer s instructions. Briefly, the genomic DNA of the assayed pups was extracted from the tail tip as template for PCR amplification. The target sites of assayed pups were amplified by PCR with specific primers (Supplementary Table 2). Around 200 ng PCR product for each site was transferred to a new tube and reannealed according to the following program: 95 ºC 10 min; 95 ºC to 85 ºC (-2.0 ºC/s); 85 ºC 1 min; 85 ºC to 75 ºC (-0.3 ºC/s); 75 ºC 1 min; 75 ºC to 65 ºC (-0.3 ºC/s); 65 ºC 1 min; 65 ºC to 55 ºC (-0.3 ºC/s); 55 ºC 1 min; 55 ºC to 45 ºC (-0.3 ºC/s); 45 ºC 1 min; 45 ºC to 35 ºC (-0.3 ºC/s); 35 ºC 1 min; 35 ºC to 25 ºC (-0.3 ºC/s); 25 ºC 1 min; 4 ºC Hold. 1 μl of the Surveyor nuclease was added to the annealed PCR product and incubated at 42ºC for 1
2 hour. Then the digested PCR product was electrophoresed on a 3% agarose gel (Takara) at 20 V/cm. The percentage of cleavage was estimated as previously described 3. DNA Sequencing of genome mutated sites The target sites were amplified by PCR with specific primers (Supplementary table 2) from genomic DNA of the mutated rats identified by Surveyor nuclease assay. The PCR products were cloned into the TA-cloning vector pmd-18t (Takara) and transformed to competent E. coli strain DH5α. After overnight culture at 37 ºC, fifteen emerged colonies were randomly picked out and sequenced. Mutations were identified by alignment of sequenced alleles to wild type allele. Off-target site screening and detection The potential off-target sites were selected based on the homology to the 8 to 12 base seed sequence at the 3 end of the sgrna because that the protospacer-adjacent motif (PAM) sequence and the 8 to 12 base seed sequence at the 3 end of the sgrna determine the DNA cleavage specificity. So higher homology at the 3 end indicates more likely to be off-targeted. Based on this rule that whole rat genome were screened for potential off-target sites for all six sgrnas. The potential off-target sites in mutant rats were amplified and subjected to Surveyor nuclease assay to further confirm whether the off-targets existed. Intracytoplasmic sperm injection and target site amplification of the embryos To quickly test the germline transmission ability of the mutations, sperm from one male double mutant male rat (by sgtet1-1 and sgtet2-1) were used. In brief, matured MII oocytes were collected from the oviduct of 7-8 week-old superovulated female SD rat, 5 µm of MG132 (Calbiochem, US) was used throughout oocytes collection to inhibit of spontaneous activation. Sperm were injected into MII oocytes using a Piezo micromanipulator. The reconstructed embryos were activated by 150 µm butyrolactone I within R1ECM medium for 2 hour, and were implanted on the same day in the oviduct of pseudo-pregnant SD female rats. Morula-stage embryos were collected for target site sequencing. To PCR amplify the target site, each embryo was placed into a PCR tube with 20 ul H2O, and was lysed by 5 heat-cool cycles (98 ºC-4 ºC). The lysate was directly used as template for PCR amplification of the target sites according to the following programs: 95 ºC 5 min; 95 ºC 30s, 59 ºC 30s, 72 ºC 30s for 60 cycles; 72 ºC 10 min. The PCR product were ligated into vector and sent for sequencing as stated above. Supplemental References 1. Hwang, W.Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nature biotechnology 31, (2013). 2. Geurts, A.M. et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325, 433 (2009). 3. Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, (2013).
3 Supplementary Discussion Determination of biallelic and monoallelic mutations with or without mosaicism of the mutant rats To determine whether a mutated rat contain a biallelic mutation or monoallelic mutation, the target site was amplified by PCR with specific primers (Supplementary Table 2). The PCR product was then cloned into vectors and transformed into E.coli competent cells. For each target site, 15~20 colonies were randomly picked for sequencing to avoid sequencing bias. Sometimes more than 2 types of alleles were recovered from single site in one rat. This is probably caused as the Cas9 based DNA cleavage may occur at later stage embryo other than the one-cell stage embryo, which leads to mosaic genotypes. Since no more than four types of alleles were recovered in each rat of all assayed rats, the cleavage mainly occurred before the two-cell-stage embryo. If no wild type allele was observed in the sequence reads, we assumed the rat had biallelic mutation, which made the rat had no wild type allele in every cell for the targeted gene. If wild type allele was observed in the sequence reads, we assumed the rat had monoallelic mutation. For both biallelic mutation and monoallelic mutation, one or two alleles were considered as non-mosaic, and more than two types of alleles were considered as mosaic. Based on these we calculated the % of biallelic mutated rats and monoallelic mutated rats. For multiple gene mutation, if all genes in the rat were biallelic mutant, the rat was counted as biallelic mutant; if it had both biallelic gene mutation and monoallelic gene mutation, the rat was counted as monoallelic mutant. The biallelic mutant or non-mosaic monoallelic mutant rats will certainly produce offspring with gene mutations. Based on our unpublished data, most of the mosaic monoallelic mutant rats will also transmit the mutations to offspring.
4 Supplementary Figures
5 Supplementary Figure 1 Tet3 gene mutation analysis in dead pups produced by injection of Cas9 enzyme together with sgtet3-1 and sgtet3-2. (a) SURVEYOR assay for indels in the PCR products for Tet3 targeted sites. Genomic DNA is extracted from newborn pups targeted by sgtet3-1 and sgtet3-2 and is then used as template for PCR amplification of the targeted sites. The wild type PCR product is 528 bp and can not be cut in the SURVEYOR assay. PCR product with Tet3- mutation is cut into 257 bp, 247 bp and 24 bp. Indel rate is calculated and labeled under the lanes with mutation. (b) Targeting site sequencing in individual mutant rat. The sgtet3-1 and sgtet3-2 target site is amplified, cloned into vectors and transformed into E.coli competent cells. To avoid sequencing bias, 15~20 colonies are randomly picked for sequencing. The mutant alleles of 7 randomly selected pups are listed in accordance with individual rat. The PAM sites are shown in red letters. In the right of each allele, the sizes of the point mutations (p), insertions (+) or deletions (Δ) are indicated between brackets. The fractions indicate the reads number of the mutant allele (numerator) out of total reads number (denominator). The reads number of wild type allele is the number difference between total reads number and total mutant reads number. The targeting locus is labeled as biallelic mutation if no wild type allele reads recovered, as monoallelic mutation if wild type allele recovered, and as mosaic if more than two types of alleles recovered (refer to Supplementary Discussion). (c) Summary of Tet3 mutant alleles observed in the sequence reads of individual rat. The number of times that each mutant allele was sequenced is shown as number.
6
7 Supplementary Figure 2 Tet1/Tet2 gene mutation analysis in rat produced by injection of Cas9 enzyme together with sgtet1-1 and sgtet2-1. (a) SURVEYOR assay for indels in the PCR products encompassing Tet1 and Tet2 targeted sites. Genomic DNA is extracted from newborn pups targeted by co-injection of sgtet1-1 and sgtet2-1 (Supplementary Table 1) and is then used as template for PCR amplification of the targeted sites. Indel rate is calculated and labeled under the lanes with mutation. The red asterisk on the gel indicate the rat has mutations in both of the the target sites. (b) Targeting site sequencing in individual mutant rat. The sgrna target sites are amplified, cloned into vectors and transformed into E.coli competent cells. To avoid sequencing bias, 10~15 colonies are randomly picked for sequencing. The mutant alleles of 4 rats are listed in accordance with individual rat. The PAM sites are shown in red letters. In the right of each allele, the sizes of the point mutations (p), insertions (+) or deletions (Δ) are indicated between brackets. The fractions indicate the reads number of the mutant allele (numerator) out of total reads number (denominator). The reads number of wild type allele is the number difference between total reads number and total mutant reads number. The targeting locus is labeled as biallelic mutation if no wild type allele reads recovered, as monoallelic mutation if wild type allele recovered, and as mosaic if more than two types of alleles recovered (refer to Supplementary Discussion). (c) Summary of Tet1 and Tet2 mutant alleles observed in the sequence reads of individual rat. The number of times that each mutant allele was sequenced is shown as number.
8
9 Supplementary Figure 3 Tet1/Tet2 gene mutation analysis in rat produced by injection of Cas9 enzyme together with sgtet1-2 and sgtet2-2. (a) SURVEYOR assay for indels in the PCR products encompassing Tet1 and Tet2 targeted sites. Genomic DNA is extracted from newborn pups targeted by co-injection of sgtet1-2 and sgtet2-2 (Supplementary Table 1) and is then used as template for PCR amplification of the targeted sites. Indel rate is calculated and labeled under the lanes with mutation. The red asterisk on the gel indicate the rat has mutations in both of the the target sites. (b) Targeting site sequencing in individual mutant rat. The sgrna target sites are amplified, cloned into vectors and transformed into E.coli competent cells. To avoid sequencing bias, 10~15 colonies are randomly picked for sequencing. The mutant alleles of 4 rats are listed in accordance with individual rat. The PAM sites are shown in red letters. In the right of each allele, the sizes of the point mutations (p), insertions (+) or deletions (Δ) are indicated between brackets. The fractions indicate the reads number of the mutant allele (numerator) out of total reads number (denominator). The reads number of wild type allele is the number difference between total reads number and total mutant reads number. The targeting locus is labeled as biallelic mutation if no wild type allele reads recovered, as monoallelic mutation if wild type allele recovered, and as mosaic if more than two types of alleles recovered (refer to Supplementary Discussion). (c) Summary of Tet1 and Tet2 mutant alleles observed in the sequence reads of individual rat. The number of times that each mutant allele was sequenced is shown as number.
10
11 Supplementary Figure 4 Sanger sequencing of targeted gene alleles in each of the triple mutated rats induced by injection of Cas9 enzyme together with sgtet1-1/sgtet2-1/sgtet3-1. The sgrna target sites are amplified, cloned into vectors and transformed into E.coli competent cells. To avoid sequencing bias, 10~15 colonies are randomly picked for sequencing. The mutant alleles of 2 rats are listed in accordance with individual rat. The PAM sites are shown in red letters. In the right of each allele, the sizes of the point mutations (p), insertions (+) or deletions (Δ) are indicated between brackets. The fractions indicate the reads number of the mutant allele (numerator) out of total reads number (denominator). The targeting locus is labeled as biallelic mutation if no wild type allele reads recovered, as monoallelic mutation if wild type allele recovered, and as mosaic if more than two types of alleles recovered (refer to Supplementary Discussion).
12
13 Supplementary Figure 5 The predicted mutant Tet3 protein sequence from the sgtet3-1 targeted mutant alleles. Four types of mutations in the Tet3 gene locus are predicted to produce truncated Tet3 protein lacking the catalytic domain.
14 Supplementary Figure 6 Detection of 5hmC levels in DNA isolated from one Tet3 mutated dead pups and two triple mutant rats by dot blot assay using anti-5hmc antibody. Genomic DNA from the rat brain is extracted and used for 5hmC dot plot. The triple mutant rat #1 had monoallelic mutation of Tet1 and Tet2, and biallelic mutation of Tet2. The triple mutant rat #2 had biallelic mutation of Tet1 and Tet2, and monoallelic mutation of Tet3. Note the reduced 5hmC level in the two triple mutant rats, with even lower 5hmC level in rat 2#. *, P < 0.05, student t test.
15
16 Supplementary Figure 7 Potential genomic off-target sites of the 6 sgrnas predicted based on the homology with the target sequence at 3 end (near PAM site). Based on the prediction method, only three sgrnas have potential off-target. The PAM sites are shown in red letters. The potential off-target sites are shown with their genomic locations.
17 Supplementary Figure 8 SURVEYOR assay for indels in the off-target sites of in tripleknockout rats. Genomic DNA is extracted from tail-tips of triple-knockout rats as template for PCR
18 amplification of the potential off-target sequences. The PCR products of mutant rats are used as positive controls. The length of PCR product and predicted cleaved fragments of the off-target are following: sgtet1-1: 1#, 494 bp, 349 bp, 145 bp; 2#, 488 bp, 256 bp, 232 bp; 3#, 459 bp, 340 bp, 119 bp; 4#, 489 bp, 259 bp, 230 bp; sgtet2-1: 1#, 418 bp, 240 bp, 178 bp; 2#, 493 bp, 316 bp, 177 bp; 3#, 451 bp, 229 bp, 222 bp; 4#, 434 bp, 264 bp, 170bp; 5#, 488 bp, 28 3bp, 205 bp; sgtet3-2: 1#, 583 bp, 355 bp, 288 bp; 2#, 429 bp, 136 bp, 293 bp. Note sgtet1-1:cas9 introduces off-targets in the predicted off-target site 3 (red asterisk).
19 Supplementary Figure 9 Sanger sequencing of targeted gene alleles in embryos produced from Tet1/Tet2 double mutant male rat and wild type female rat. Male Tet1/Tet2 double mutant rat sperm fertilized wild type oocytes by intracytoplasmic sperm injection (ICSI). The resulted embryos were assayed for transmission of Tet1/Tet2 mutation from the mutant rat by PCR and sequencing. Of 7 embryos successfully assayed by Sanger sequencing, five inherited mutations in the Tet2 site only, and 2 inherited mutations in the both the Tet1 and Tet2 sites (embryo 1 and 2), indicating the mutations in the founder rats could be transmitted to next generation.
20 Supplementary Tables Supplementary Table 1. The designed sgrna target sites for Tet1, Tet2 and Tet3 genes sgr NAs sgte t1-1 sgte t1-2 sgte t2-1 sgte t2-2 sgte t3-1 sgte t3-2 Targeted site (5 3 )(PAM is underlined) GTGGAATATGAAG ACATTGCTGG GAAGAGGGGCGTC CTTTCTCTGG ACCTGATGCCTACA GGAATCAGG CGAGCTATAGAATG CCGTTTGGG TGCTCCCCTATACA AGCGGCTGG ACCCCAGGCCTATC AGAACCAGG The underline nucleotides represent PAM sequence; the BsaI digested overhang sequences are highlighted in yellow and green, respectively. Oligonucleotide 1 (5-3 ) TAGGGTGGAATATG AAGACATTGC TAGGGAAGAGGGG CGTCCTTTCTC TAGGACCTGATGCC TACAGGAATC TAGGCGAGCTATAG AATGCCGTTT TAGGTGCTCCCCTA TACAAGCGGC TAGGACCCCAGGCC TATCAGAACC Oligonucleotide 2 (5-3 ) AAACGCAATGTCTT CATATTCCAC AAACGAGAAAGGA CGCCCCTCTTC AAACGATTCCTGTA GGCATCAGGT AAACAAACGGCATT CTATAGCTCG AAACGCCGCTTGTA TAGGGGAGCA AAACGGTTCTGATA GGCCTGGGGT Supplementary Table 2. The ratios of each kind of mutation type in the mutant rats*. Injected sgrnas sgtet3-1 /sgtet3-2 sgtet1-1 /sgtet2-1 sgtet1-2 /sgtet2-2 sgtet1-1 /sgtet2-1 /sgtet3-1 No. of assayed Assayed gene Biallelic Monoallelic mutated rats Nonmosaic (%) Mosaic (%) Nonmosaic (%) Mosaic (%) 18 Tet (27.8) 0 0 (72.2) 15 Tet1 1 (6.7) (26.6) (66.7) Tet2 8 (53.3) 3 (20.0) 2 (13.3) 2 (13.3) Tet1/Tet (60.0) 6 (40.0) 11 Tet (9.1) 0 (90.9) Tet2 4 (36.4) 1 (9.1) 3 (27.3) 3 (27.3) Tet1/Tet2 4 (36.3) 1 (9.1) 3 (27.3) 3 (27.3) 13 Tet1 1 (7.7) 0 7 (53.8) 5 (38.5) Tet (15.4) 0 0 (84.6) Tet (23.1) (76.9) Tet1/Tet2/Tet (61.5) 5 (38.5) *Please refer to the Supplementary Discussion for mutation type determination.
21 Supplementary Table 3. Primer list for PCR amplification of the targeted sites. Primer name Target site Primer sequence (5-3 ) Product length Annealing temperature D-rTet1-F Tet1 (site1) TTGCCTTCCAGCCTTGATACAC 472 bp 59 D-rTet1-R Tet1 (site2) ACCACAGTGCTTCCATTGTTCA D-rTet2-1F Tet2 (site1) ACCCAAACCCAAAGAGAGAACG 400 bp 65 D-rTet2-1R AGTCAATCCAGGACAGCCAAGA D-rTet2-2F Tet2 (site2) GGACCTGGCAGTGTAAGTCAGT 409 bp 59 D-rTet2-2R GCTTGTGCTTCGCTGGTAGAG D-rTet3-F Tet3 (site1) TTCCTGGTGAGCCTCAGTATCC 528 bp 59 D-rTet3-R Tet3 (site2) GCTTGCTTGCCTAGACAGACAG
22 Supplementary Sequences The underline sequences present the Cas9 sequence; Italic letters in green color represent the sgrna sequence; yellow highlighted sequences represent spacer sequence, red letters present the enzyme cutting site. >peasy-t7-cas9-sv40 polya TAATACGACTCACTATAGGGCGAATTGGGCCCTCTAGAGCTAGCATGGACTATAAGGACCACGACGGA GACTACAAGGATCATGATATTGATTACAAAGACGATGACGATAAGATGGCCCCAAAGAAGAAGCGGAA GGTCGGTATCCACGGAGTCCCAGCAGCCGACAAGAAGTACAGCATCGGCCTGGACATCGGCACCAAC TCTGTGGGCTGGGCCGTGATCACCGACGAGTACAAGGTGCCCAGCAAGAAATTCAAGGTGCTGGGCA ACACCGACCGGCACAGCATCAAGAAGAACCTGATCGGAGCCCTGCTGTTCGACAGCGGCGAAACAG CCGAGGCCACCCGGCTGAAGAGAACCGCCAGAAGAAGATACACCAGACGGAAGAACCGGATCTGCT ATCTGCAAGAGATCTTCAGCAACGAGATGGCCAAGGTGGACGACAGCTTCTTCCACAGACTGGAAGA GTCCTTCCTGGTGGAAGAGGATAAGAAGCACGAGCGGCACCCCATCTTCGGCAACATCGTGGACGAG GTGGCCTACCACGAGAAGTACCCCACCATCTACCACCTGAGAAAGAAACTGGTGGACAGCACCGACA AGGCCGACCTGCGGCTGATCTATCTGGCCCTGGCCCACATGATCAAGTTCCGGGGCCACTTCCTGATC GAGGGCGACCTGAACCCCGACAACAGCGACGTGGACAAGCTGTTCATCCAGCTGGTGCAGACCTACA ACCAGCTGTTCGAGGAAAACCCCATCAACGCCAGCGGCGTGGACGCCAAGGCCATCCTGTCTGCCAG ACTGAGCAAGAGCAGACGGCTGGAAAATCTGATCGCCCAGCTGCCCGGCGAGAAGAAGAATGGCCT GTTCGGCAACCTGATTGCCCTGAGCCTGGGCCTGACCCCCAACTTCAAGAGCAACTTCGACCTGGCC GAGGATGCCAAACTGCAGCTGAGCAAGGACACCTACGACGACGACCTGGACAACCTGCTGGCCCAG ATCGGCGACCAGTACGCCGACCTGTTTCTGGCCGCCAAGAACCTGTCCGACGCCATCCTGCTGAGCGA CATCCTGAGAGTGAACACCGAGATCACCAAGGCCCCCCTGAGCGCCTCTATGATCAAGAGATACGACG AGCACCACCAGGACCTGACCCTGCTTAAGGCTCTCGTGCGGCAGCAGCTGCCTGAGAAGTACAAAGA GATTTTCTTCGACCAGAGCAAGAACGGCTACGCCGGCTACATTGACGGCGGAGCCAGCCAGGAAGAG TTCTACAAGTTCATCAAGCCCATCCTGGAAAAGATGGACGGCACCGAGGAACTGCTCGTGAAGCTGA ACAGAGAGGACCTGCTGCGGAAGCAGCGGACCTTCGACAACGGCAGCATCCCCCACCAGATCCACCT GGGAGAGCTGCACGCCATTCTGCGGCGGCAGGAAGATTTTTACCCATTCCTGAAGGACAACCGGGAA AAGATCGAGAAGATCCTGACCTTCCGCATCCCCTACTACGTGGGCCCTCTGGCCAGGGGAAACAGCA GATTCGCCTGGATGACCAGAAAGAGCGAGGAAACCATCACCCCCTGGAACTTCGAGGAAGTGGTGGA CAAGGGCGCTTCCGCCCAGAGCTTCATCGAGCGGATGACCAACTTCGATAAGAACCTGCCCAACGAG AAGGTGCTGCCCAAGCACAGCCTGCTGTACGAGTACTTCACCGTGTATAACGAGCTGACCAAAGTGA AATACGTGACCGAGGGAATGAGAAAGCCCGCCTTCCTGAGCGGCGAGCAGAAAAAGGCCATCGTGG ACCTGCTGTTCAAGACCAACCGGAAAGTGACCGTGAAGCAGCTGAAAGAGGACTACTTCAAGAAAAT CGAGTGCTTCGACTCCGTGGAAATCTCCGGCGTGGAAGATCGGTTCAACGCCTCCCTGGGCACATACC ACGATCTGCTGAAAATTATCAAGGACAAGGACTTCCTGGACAATGAGGAAAACGAGGACATTCTGGA AGATATCGTGCTGACCCTGACACTGTTTGAGGACAGAGAGATGATCGAGGAACGGCTGAAAACCTATG CCCACCTGTTCGACGACAAAGTGATGAAGCAGCTGAAGCGGCGGAGATACACCGGTTGGGGCAGGCT GAGCCGGAAGCTGATCAACGGCATCCGGGACAAGCAGTCCGGCAAGACAATCCTGGATTTCCTGAAG TCCGACGGCTTCGCCAACAGAAACTTCATGCAGCTGATCCACGACGACAGCCTGACCTTTAAAGAGG ACATCCAGAAAGCCCAGGTGTCCGGCCAGGGCGATAGCCTGCACGAGCACATTGCCAATCTGGCCGG CAGCCCCGCCATTAAGAAGGGCATCCTGCAGACAGTGAAGGTGGTGGACGAGCTCGTGAAAGTGATG GGCCGGCACAAGCCCGAGAACATCGTGATCGAAATGGCCAGAGAGAACCAGACCACCCAGAAGGGA CAGAAGAACAGCCGCGAGAGAATGAAGCGGATCGAAGAGGGCATCAAAGAGCTGGGCAGCCAGATC
23 CTGAAAGAACACCCCGTGGAAAACACCCAGCTGCAGAACGAGAAGCTGTACCTGTACTACCTGCAGA ATGGGCGGGATATGTACGTGGACCAGGAACTGGACATCAACCGGCTGTCCGACTACGATGTGGACCAT ATCGTGCCTCAGAGCTTTCTGAAGGACGACTCCATCGACAACAAGGTGCTGACCAGAAGCGACAAGA ACCGGGGCAAGAGCGACAACGTGCCCTCCGAAGAGGTCGTGAAGAAGATGAAGAACTACTGGCGGC AGCTGCTGAACGCCAAGCTGATTACCCAGAGAAAGTTCGACAATCTGACCAAGGCCGAGAGAGGCGG CCTGAGCGAACTGGATAAGGCCGGCTTCATCAAGAGACAGCTGGTGGAAACCCGGCAGATCACAAAG CACGTGGCACAGATCCTGGACTCCCGGATGAACACTAAGTACGACGAGAATGACAAGCTGATCCGGG AAGTGAAAGTGATCACCCTGAAGTCCAAGCTGGTGTCCGATTTCCGGAAGGATTTCCAGTTTTACAAA GTGCGCGAGATCAACAACTACCACCACGCCCACGACGCCTACCTGAACGCCGTCGTGGGAACCGCCC TGATCAAAAAGTACCCTAAGCTCGAGAGCGAGTTCGTGTACGGCGACTACAAGGTGTACGACGTGCG GAAGATGATCGCCAAGAGCGAGCAGGAAATCGGCAAGGCTACCGCCAAGTACTTCTTCTACAGCAAC ATCATGAACTTTTTCAAGACCGAGATTACCCTGGCCAACGGCGAGATCCGGAAGCGGCCTCTGATCGA GACAAACGGCGAAACCGGGGAGATCGTGTGGGATAAGGGCCGGGATTTTGCCACCGTGCGGAAAGTG CTGAGCATGCCCCAAGTGAATATCGTGAAAAAGACCGAGGTGCAGACAGGCGGCTTCAGCAAAGAGT CTATCCTGCCCAAGAGGAACAGCGATAAGCTGATCGCCAGAAAGAAGGACTGGGACCCTAAGAAGTA CGGCGGCTTCGACAGCCCCACCGTGGCCTATTCTGTGCTGGTGGTGGCCAAAGTGGAAAAGGGCAAG TCCAAGAAACTGAAGAGTGTGAAAGAGCTGCTGGGGATCACCATCATGGAAAGAAGCAGCTTCGAG AAGAATCCCATCGACTTTCTGGAAGCCAAGGGCTACAAAGAAGTGAAAAAGGACCTGATCATCAAGC TGCCTAAGTACTCCCTGTTCGAGCTGGAAAACGGCCGGAAGAGAATGCTGGCCTCTGCCGGCGAACT GCAGAAGGGAAACGAACTGGCCCTGCCCTCCAAATATGTGAACTTCCTGTACCTGGCCAGCCACTATG AGAAGCTGAAGGGCTCCCCCGAGGATAATGAGCAGAAACAGCTGTTTGTGGAACAGCACAAGCACTA CCTGGACGAGATCATCGAGCAGATCAGCGAGTTCTCCAAGAGAGTGATCCTGGCCGACGCTAATCTGG ACAAAGTGCTGTCCGCCTACAACAAGCACCGGGATAAGCCCATCAGAGAGCAGGCCGAGAATATCAT CCACCTGTTTACCCTGACCAATCTGGGAGCCCCTGCCGCCTTCAAGTACTTTGACACCACCATCGACC GGAAGAGGTACACCAGCACCAAAGAGGTGCTGGACGCCACCCTGATCCACCAGAGCATCACCGGCCT GTACGAGACACGGATCGACCTGTCTCAGCTGGGAGGCGACAAGCGTCCTGCTGCTACTAAGAAAGCT GGTCAAGCTAAGAAAAAGAAATAAGAATTCGGATCCAATCAACCTCTGGATTACAAAATTTGTGAAAG ATTGACTGGTATTCTTAACTATGTTGCTCCTTTTACGCTATGTGGATACGCTGCTTTAATGCCTTTGTATC ATGCGTTGTTTATTGCAGCTTATAATGGTTACAAATAAAGCAATAGCATCACAAATTTCACAAATAAAGC ATTTTTTTCACTGCATTCTAGTTGTGGTTTGTCCAAACTCATCAATGTATCTTAAAGCTTGGCGTAATCAT GGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAATTCCACACAACATACGAGCCGGAAGCA TAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTCACATTAATTGCGTTGCGCTCACTGCCC GCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATGAATCGGCCAACGCGCGGGGAGAGGCG GTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGC GAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAA GAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTC CATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGA CAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTG CCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCACGCTGT AGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCC CGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACT GGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAGTTCTTGAAG TGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGTTACC TTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGTTTTTTTGT
24 TTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAGAAGATCCTTTGATCTTTTCTACGGGGT CTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTC ACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCTAAAGTATATATGAGTAAACTTGGTCTG ACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGATCTGTCTATTTCGTTCATCCATAGTTGC CTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATCTGGCCCCAGTGCTGCAATGAT ACCGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCAATAAACCAGCCAGCCGGAAGGGCCGAG CGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCTATTAATTGTTGCCGGGAAGCTAGAGTA AGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATTGCTACAGGCATCGTGGTGTCACGCTCG TCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCAAGGCGAGTTACATGATCCCCCATGTTG TGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGTGTTATC ACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATGCCATCCGTAAGATGCTTTTCTGTGACT GGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCTCTTGCCCGGCGTC AATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGTGCTCATCATTGGAAAACGTTCTTCGG GGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTTCGATGTAACCCACTCGTGCACCCAAC TGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAGCAAAAACAGGAAGGCAAAATGCCGC AAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCATACTCTTCCTTTTTCAATTCAGAAGAA CTCGTCAAGAAGGCGATAGAAGGCGATGCGCTGCGAATCGGGAGCGGCGATACCGTAAAGCACGAGG AAGCGGTCAGCCCATTCGCCGCCAAGCTCTTCAGCAATATCACGGGTAGCCAACGCTATGTCCTGATA GCGGTCCGCCACACCCAGCCGGCCACAGTCGATGAATCCAGAAAAGCGGCCATTTTCCACCATGATAT TCGGCAAGCAGGCATCGCCATGGGTCACGACGAGATCCTCGCCGTCGGGCATGCGCGCCTTGAGCCT GGCGAACAGTTCGGCTGGCGCGAGCCCCTGATGCTCTTCGTCCAGATCATCCTGATCGACAAGACCGG CTTCCATCCGAGTACGTGCTCGCTCGATGCGATGTTTCGCTTGGTGGTCGAATGGGCAGGTAGCCGGAT CAAGCGTATGCAGCCGCCGCATTGCATCAGCCATGATGGATACTTTCTCGGCAGGAGCAAGGTGGGAT GACAGGAGATCCTGCCCCGGCACTTCGCCCAATAGCAGCCAGTCCCTTCCCGCTTCAGTGACAACGTC GAGCACAGCTGCGCAAGGAACGCCCGTCGTGGCCAGCCACGATAGCCGCGCTGCCTCGTCCTGCAGT TCATTCAGGGCACCGGACAGGTCGGTCTTGACAAAAAGAACCGGGCGCCCCTGCGCTGACAGCCGGA ACACGGCGGCATCAGAGCAGCCGATTGTCTGTTGTGCCCAGTCATAGCCGAATAGCCTCTCCACCCAA GCGGCCGGAGAACCTGCGTGCAATCCATCTTGTTCAATCATGCGAAACGATCCTCATCCTGTCTCTTGA TCAGATCTTGATCCCCTGCGCCATCAGATCCTTGGCGGCAAGAAAGCCATCCAGTTTACTTTGCAGGGC TTCCCAACCTTACCAGAGGGCGCCCCAGCTGGCAATTCCGGTTCGCTTGCTGTCCATAAAACCGCCCA GTCTAGCTATCGCCATGTAAGCCCACTGCAAGCTACCTGCTTTCTCTTTGCGCTTGCGTTTTCCCTTGTC CAGATAGCCCAGTAGCTGACATTCATCCGGGGTCAGCACCGTTTCTGCGGACTGGCTTTCTACGTGTTC CGCTTCCTTTAGCAGCCCTTGCGCCCTGAATTTTGTTAAAATTCGCGTTAAATTTTTGTTAAATCAGCTC ATTTTTTAACCAATAGGCCGAAATCGGCAAAATCCCTTATAAATCAAAAGAATAGACCGAGATAGGGTT GAGTGTTGTTCCAGTTTGGAACAAGAGTCCACTATTAAAGAACGTGGACTCCAACGTCAAAGGGCGA AAAACCGTCTATCAGGGCGATGGCCCACTACGTGAACCATCACCCTAATCAAGTTTTTTGGGGTCGAG GTGCCGTAAAGCACTAAATCGGAACCCTAAAGGGAGCCCCCGATTTAGAGCTTGACGGGGAAAGCCG GCGAACGTGGCGAGAAAGGAAGGGAAGAAAGCGAAAGGAGCGGGCGCTAGGGCGCTGGCAAGTGT AGCGGTCACGCTGCGCGTAACCACCACACCCGCCGCGCTTAATGCGCCGCTACAGGGCGCGTCCATTC GCCATTCAGGCTGCGCAACTGTTGGGAAGGGCGATCGGTGCGGGCCTCTTCGCTATTACGCCAGCTGG CGAAAGGGGGATGTGCTGCAAGGCGATTAAGTTGGGTAACGCCAGGGTTTTCCCAGTCACGACGTTG TAAAACGACGGCCAGTGAATTG >puc19-t7-sgrna-tet1-1
25 TAATACGACTCACTATAGGGTGGAATATGAAGACATTGCGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGG CTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTATAAGGGGATCCTCTAGAGTCGACCT GCAGGCATGCAAGCTTGGCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAA TTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTC ACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATGA ATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTC GCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCA CAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTA AAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGC TCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCC TCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCG TGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCT GTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAAC CCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGT AGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTA TCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACC ACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAG AAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTG GTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCT AAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGA TCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTT ACCATCTGGCCCCAGTGCTGCAATGATACCGCGTGAGCCACGCTCACCGGCTCCAGATTTATCAGCAAT AAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCT ATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATT GCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCA AGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTC AGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATG CCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGG CGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGT GCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTT CGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAG CAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCA TACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAA TGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCTA AGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGCGT TTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAAGC GGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGGCT TAACTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCATATGCGGTGTGAAATACCGCACAGAT GCGTAAGGAGAAAATACCGCATCAGGCGCCATTCGCCATTCAGGCTGCGCAACTGTTGGGAAGGGCG ATCGGTGCGGGCCTCTTCGCTATTACGCCAGCTGGCGAAAGGGGGATGTGCTGCAAGGCGATTAAGTT GGGTAACGCCAGGGTTTTCCCAGTCACGACGTTGTAAAACGACGGCCAGTGAATTCGAGCTCGGTAC CC >puc19-t7-sgrna-tet1-2
26 TAATACGACTCACTATAGGGAAGAGGGGCGTCCTTTCTCGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAG GCTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTTAAAGGGGATCCTCTAGAGTCGAC CTGCAGGCATGCAAGCTTGGCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCAC AATTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAAC TCACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAAT GAATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGAC TCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATC CACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCG TAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGAC GCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTC CCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAG CGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGG CTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCA ACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTA TGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTG GTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAA ACCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCA AGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTT TGGTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAAT CTAAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGC GATCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGC TTACCATCTGGCCCCAGTGCTGCAATGATACCGCGTGAGCCACGCTCACCGGCTCCAGATTTATCAGCA ATAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTC TATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCAT TGCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATC AAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGT CAGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCAT GCCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCG GCGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAG TGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGT TCGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGA GCAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTC ATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGA ATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCT AAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGC GTTTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAA GCGGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGG CTTAACTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCATATGCGGTGTGAAATACCGCACAG ATGCGTAAGGAGAAAATACCGCATCAGGCGCCATTCGCCATTCAGGCTGCGCAACTGTTGGGAAGGGC GATCGGTGCGGGCCTCTTCGCTATTACGCCAGCTGGCGAAAGGGGGATGTGCTGCAAGGCGATTAAGT TGGGTAACGCCAGGGTTTTCCCAGTCACGACGTTGTAAAACGACGGCCAGTGAATTCGAGCTCGGTA CCC >puc19-t7-sgrna-tet2-1
27 TAATACGACTCACTATAGGACCTGATGCCTACAGGAATCGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGG CTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTATAAGGGGATCCTCTAGAGTCGACCT GCAGGCATGCAAGCTTGGCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAA TTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTC ACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATGA ATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTC GCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCA CAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTA AAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGC TCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCC TCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCG TGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCT GTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAAC CCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGT AGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTA TCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACC ACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAG AAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTG GTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCT AAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGA TCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTT ACCATCTGGCCCCAGTGCTGCAATGATACCGCGTGAGCCACGCTCACCGGCTCCAGATTTATCAGCAAT AAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCT ATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATT GCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCA AGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTC AGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATG CCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGG CGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGT GCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTT CGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAG CAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCA TACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAA TGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCTA AGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGCGT TTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAAGC GGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGGCT TAACTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCATATGCGGTGTGAAATACCGCACAGAT GCGTAAGGAGAAAATACCGCATCAGGCGCCATTCGCCATTCAGGCTGCGCAACTGTTGGGAAGGGCG ATCGGTGCGGGCCTCTTCGCTATTACGCCAGCTGGCGAAAGGGGGATGTGCTGCAAGGCGATTAAGTT GGGTAACGCCAGGGTTTTCCCAGTCACGACGTTGTAAAACGACGGCCAGTGAATTCGAGCTCGGTAC CC >puc19-t7-sgrna-tet2-2
28 TAATACGACTCACTATAGGCGAGCTATAGAATGCCGTTTGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGG CTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTATAAGGGGATCCTCTAGAGTCGACCT GCAGGCATGCAAGCTTGGCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAA TTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTC ACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATGA ATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTC GCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCA CAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTA AAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGC TCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCC TCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCG TGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCT GTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAAC CCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGT AGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTA TCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACC ACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAG AAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTG GTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCT AAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGA TCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTT ACCATCTGGCCCCAGTGCTGCAATGATACCGCGTGAGCCACGCTCACCGGCTCCAGATTTATCAGCAAT AAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCT ATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATT GCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCA AGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTC AGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATG CCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGG CGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGT GCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTT CGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAG CAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCA TACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAA TGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCTA AGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGCGT TTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAAGC GGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGGCT TAACTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCATATGCGGTGTGAAATACCGCACAGAT GCGTAAGGAGAAAATACCGCATCAGGCGCCATTCGCCATTCAGGCTGCGCAACTGTTGGGAAGGGCG ATCGGTGCGGGCCTCTTCGCTATTACGCCAGCTGGCGAAAGGGGGATGTGCTGCAAGGCGATTAAGTT GGGTAACGCCAGGGTTTTCCCAGTCACGACGTTGTAAAACGACGGCCAGTGAATTCGAGCTCGGTAC CC >puc19-t7-sgrna-tet3-1
29 TAATACGACTCACTATAGGTGCTCCCCTATACAAGCGGCGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGG CTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTATAAGGGGATCCTCTAGAGTCGACCT GCAGGCATGCAAGCTTGGCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAA TTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTC ACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATGA ATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTC GCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCA CAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTA AAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGC TCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCC TCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCG TGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCT GTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAAC CCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGT AGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTA TCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACC ACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAG AAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTG GTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCT AAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGA TCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTT ACCATCTGGCCCCAGTGCTGCAATGATACCGCGTGAGCCACGCTCACCGGCTCCAGATTTATCAGCAAT AAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCT ATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATT GCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCA AGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTC AGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATG CCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGG CGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGT GCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTT CGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAG CAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCA TACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAA TGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCTA AGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGCGT TTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAAGC GGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGGCT TAACTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCATATGCGGTGTGAAATACCGCACAGAT GCGTAAGGAGAAAATACCGCATCAGGCGCCATTCGCCATTCAGGCTGCGCAACTGTTGGGAAGGGCG ATCGGTGCGGGCCTCTTCGCTATTACGCCAGCTGGCGAAAGGGGGATGTGCTGCAAGGCGATTAAGTT GGGTAACGCCAGGGTTTTCCCAGTCACGACGTTGTAAAACGACGGCCAGTGAATTCGAGCTCGGTAC CC >puc19-t7-sgrna-tet3-2
30 TAATACGACTCACTATAGGACCCCAGGCCTATCAGAACCGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAG GCTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTATAAGGGGATCCTCTAGAGTCGACC TGCAGGCATGCAAGCTTGGCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACA ATTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACT CACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATG AATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACT CGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCC ACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGT AAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACG CTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCC CTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGC GTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGC TGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAA CCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTAT GTAGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGG TATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAA CCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAA GAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTT GGTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATC TAAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCG ATCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCT TACCATCTGGCCCCAGTGCTGCAATGATACCGCGTGAGCCACGCTCACCGGCTCCAGATTTATCAGCA ATAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTC TATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCAT TGCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATC AAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGT CAGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCAT GCCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCG GCGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAG TGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGT TCGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGA GCAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTC ATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGA ATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCT AAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGC GTTTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAA GCGGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGG CTTAACTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCATATGCGGTGTGAAATACCGCACAG ATGCGTAAGGAGAAAATACCGCATCAGGCGCCATTCGCCATTCAGGCTGCGCAACTGTTGGGAAGGGC GATCGGTGCGGGCCTCTTCGCTATTACGCCAGCTGGCGAAAGGGGGATGTGCTGCAAGGCGATTAAGT TGGGTAACGCCAGGGTTTTCCCAGTCACGACGTTGTAAAACGACGGCCAGTGAATTCGAGCTCGGTA CCC
Protocol for Genome Editing via the RNA-guided Cas9 Nuclease in. Zebrafish Embryos 1
 Protocol for Genome Editing via the RNA-guided Cas9 Nuclease in Zebrafish Embryos 1 1. In vitro synthesis of capped Cas9 mrna The full length of humanized Cas9 cdnas with double NLS were cloned into pxt7
Protocol for Genome Editing via the RNA-guided Cas9 Nuclease in Zebrafish Embryos 1 1. In vitro synthesis of capped Cas9 mrna The full length of humanized Cas9 cdnas with double NLS were cloned into pxt7
Transfection of CRISPR/Cas9 Nuclease NLS ribonucleoprotein (RNP) into adherent mammalian cells using Lipofectamine RNAiMAX
 Transfection of CRISPR/Cas9 Nuclease NLS ribonucleoprotein (RNP) into adherent mammalian cells using Lipofectamine RNAiMAX INTRODUCTION The CRISPR/Cas genome editing system consists of a single guide RNA
Transfection of CRISPR/Cas9 Nuclease NLS ribonucleoprotein (RNP) into adherent mammalian cells using Lipofectamine RNAiMAX INTRODUCTION The CRISPR/Cas genome editing system consists of a single guide RNA
Supplementary Materials
 Supplementary Materials Supplementary Methods Supplementary Discussion Supplementary Figure 1 Calculated frequencies of embryo cells bearing bi-allelic alterations. Targeted indel mutations induced by
Supplementary Materials Supplementary Methods Supplementary Discussion Supplementary Figure 1 Calculated frequencies of embryo cells bearing bi-allelic alterations. Targeted indel mutations induced by
CRISPR/Cas9 Gene Editing Tools
 CRISPR/Cas9 Gene Editing Tools - Guide-it Products for Successful CRISPR/Cas9 Gene Editing - Why choose Guide-it products? Optimized methods designed for speed and ease of use Complete kits that don t
CRISPR/Cas9 Gene Editing Tools - Guide-it Products for Successful CRISPR/Cas9 Gene Editing - Why choose Guide-it products? Optimized methods designed for speed and ease of use Complete kits that don t
Simple protocol for gene editing using GenCrisprTM Cas9 nuclease
 Simple protocol for gene editing using GenCrisprTM Cas9 nuclease Contents Protocol Step 1: Choose the target DNA sequence Step 2: Design sgrna Step 3: Preparation for sgrna 3.1 In vitro transcription of
Simple protocol for gene editing using GenCrisprTM Cas9 nuclease Contents Protocol Step 1: Choose the target DNA sequence Step 2: Design sgrna Step 3: Preparation for sgrna 3.1 In vitro transcription of
Bart Williams, PhD Van Andel Research Center
 A History of Genome Editing in the Laboratory Implications for Translational Applications Bart Williams, PhD Van Andel Research Center Introduction by Matthew Denenberg, MD DeVos Childrens Hospital Disclosures:
A History of Genome Editing in the Laboratory Implications for Translational Applications Bart Williams, PhD Van Andel Research Center Introduction by Matthew Denenberg, MD DeVos Childrens Hospital Disclosures:
CRISPR/Cas9 Gene Editing Tools
 CRISPR/Cas9 Gene Editing Tools - Separations Simply Spectacular INDELS Identify indels Determine if one or both copies of your gene have indels The Guide-it Genotype Confirmation Kit: Simple detection
CRISPR/Cas9 Gene Editing Tools - Separations Simply Spectacular INDELS Identify indels Determine if one or both copies of your gene have indels The Guide-it Genotype Confirmation Kit: Simple detection
Guide-it Indel Identification Kit User Manual
 Clontech Laboratories, Inc. Guide-it Indel Identification Kit User Manual Cat. No. 631444 (120114) Clontech Laboratories, Inc. A Takara Bio Company 1290 Terra Bella Avenue, Mountain View, CA 94043, USA
Clontech Laboratories, Inc. Guide-it Indel Identification Kit User Manual Cat. No. 631444 (120114) Clontech Laboratories, Inc. A Takara Bio Company 1290 Terra Bella Avenue, Mountain View, CA 94043, USA
Supplementary Information. Optimization of the production of knock-in alleles by CRISPR/Cas9 microinjection into the mouse zygote
 Supplementary Information Supplementary Figures S1 to S5 and Tables S1 to S3. Optimization of the production of knock-in alleles by CRISPR/Cas9 microinjection into the mouse zygote Aurélien Raveux, Sandrine
Supplementary Information Supplementary Figures S1 to S5 and Tables S1 to S3. Optimization of the production of knock-in alleles by CRISPR/Cas9 microinjection into the mouse zygote Aurélien Raveux, Sandrine
Protocols for cell lines using CRISPR/CAS
 Protocols for cell lines using CRISPR/CAS Procedure overview Map Preparation of CRISPR/CAS plasmids Expression vectors for guide RNA (U6-gRNA) and Cas9 gene (CMV-p-Cas9) are ampicillin-resist ant and stable
Protocols for cell lines using CRISPR/CAS Procedure overview Map Preparation of CRISPR/CAS plasmids Expression vectors for guide RNA (U6-gRNA) and Cas9 gene (CMV-p-Cas9) are ampicillin-resist ant and stable
Puro. Knockout Detection (KOD) Kit
 Puro Knockout Detection (KOD) Kit Cat. No. CC-03 18 Oct. 2016 Contents I. Kit Contents and Storage II. Product Overview III. Methods Experimental Outline Genomic DNA Preparation Obtain Hybrid DNA Digest
Puro Knockout Detection (KOD) Kit Cat. No. CC-03 18 Oct. 2016 Contents I. Kit Contents and Storage II. Product Overview III. Methods Experimental Outline Genomic DNA Preparation Obtain Hybrid DNA Digest
Guide-it sgrna In Vitro Transcription and Screening Systems User Manual
 Clontech Laboratories, Inc. Guide-it sgrna In Vitro Transcription and Screening Systems User Manual Cat. Nos. 631438, 631439 & 631440 (042114) Clontech Laboratories, Inc. A Takara Bio Company 1290 Terra
Clontech Laboratories, Inc. Guide-it sgrna In Vitro Transcription and Screening Systems User Manual Cat. Nos. 631438, 631439 & 631440 (042114) Clontech Laboratories, Inc. A Takara Bio Company 1290 Terra
Supplementary Methods pcfd5 cloning protocol
 Supplementary Methods cloning protocol vermilion trna grna trna grna U6:3 Terminator AmpR attb is a vector for expressing one or multiple trna-flanked Cas9 grnas under the control of the strong, ubiquitous
Supplementary Methods cloning protocol vermilion trna grna trna grna U6:3 Terminator AmpR attb is a vector for expressing one or multiple trna-flanked Cas9 grnas under the control of the strong, ubiquitous
Nature Biotechnology: doi: /nbt.4166
 Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
Guide-it sgrna In Vitro Transcription and Screening Systems User Manual
 Guide-it sgrna In Vitro Transcription and Screening Systems User Manual Cat. Nos. 632638, 632639, 632635, 632636, 632637 (040618) 1290 Terra Bella Avenue, Mountain View, CA 94043, USA U.S. Technical Support:
Guide-it sgrna In Vitro Transcription and Screening Systems User Manual Cat. Nos. 632638, 632639, 632635, 632636, 632637 (040618) 1290 Terra Bella Avenue, Mountain View, CA 94043, USA U.S. Technical Support:
CRISPR/Cas9 Genome Editing: Transfection Methods
 CRISPR/ Genome Editing: Transfection Methods For over 20 years Mirus Bio has developed and manufactured high performance transfection products and technologies. That expertise is now being applied to the
CRISPR/ Genome Editing: Transfection Methods For over 20 years Mirus Bio has developed and manufactured high performance transfection products and technologies. That expertise is now being applied to the
SUPPLEMENTARY INFORMATION
 Gene replacements and insertions in rice by intron targeting using CRISPR Cas9 Table of Contents Supplementary Figure 1. sgrna-induced targeted mutations in the OsEPSPS gene in rice protoplasts. Supplementary
Gene replacements and insertions in rice by intron targeting using CRISPR Cas9 Table of Contents Supplementary Figure 1. sgrna-induced targeted mutations in the OsEPSPS gene in rice protoplasts. Supplementary
Supplementary Figure 1. Diagram for CATCHA construct. Nature Biotechnology doi: /nbt.3444
 Supplementary Figure 1 Diagram for CATCHA construct. Supplementary Figure 2 Representative view of ebony (left) and non-ebony (right) F2 flies from experiments described in Fig. 1c. F0 #1 F0 #2 F0 #3 F0
Supplementary Figure 1 Diagram for CATCHA construct. Supplementary Figure 2 Representative view of ebony (left) and non-ebony (right) F2 flies from experiments described in Fig. 1c. F0 #1 F0 #2 F0 #3 F0
Genome editing. Knock-ins
 Genome editing Knock-ins Experiment design? Should we even do it? In mouse or rat, the HR-mediated knock-in of homologous fragments derived from a donor vector functions well. However, HR-dependent knock-in
Genome editing Knock-ins Experiment design? Should we even do it? In mouse or rat, the HR-mediated knock-in of homologous fragments derived from a donor vector functions well. However, HR-dependent knock-in
SUPPLEMENT MATERIALS FOR CURTIN,
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 SUPPLEMENT MATERIALS FOR CURTIN, et al. Validating genome-wide association candidates: Selecting, testing, and characterizing
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 SUPPLEMENT MATERIALS FOR CURTIN, et al. Validating genome-wide association candidates: Selecting, testing, and characterizing
CELLTECHGEN For Research Only. Construction of all-in-one vector for Lenti-virus system (Example: Lenti-EF1 -Cas9-EGFP-U6 sgrna vector)
 Construction of all-in-one vector for Lenti-virus system (Example: Lenti-EF1 -Cas9-EGFP-U6 sgrna vector) Catalog number: CTG-CAS9-18 Introduction The vector Lenti-EF1 -Cas9-EGFP-U6 sgrna is designed for
Construction of all-in-one vector for Lenti-virus system (Example: Lenti-EF1 -Cas9-EGFP-U6 sgrna vector) Catalog number: CTG-CAS9-18 Introduction The vector Lenti-EF1 -Cas9-EGFP-U6 sgrna is designed for
Generation of App knock-in mice reveals deletion mutations protective against Alzheimer s. disease-like pathology. Nagata et al.
 Generation of App knock-in mice reveals deletion mutations protective against Alzheimer s disease-like pathology Nagata et al. Supplementary Fig 1. Previous App knock-in model did not show Aβ accumulation
Generation of App knock-in mice reveals deletion mutations protective against Alzheimer s disease-like pathology Nagata et al. Supplementary Fig 1. Previous App knock-in model did not show Aβ accumulation
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.aaa5945/dc1 Supplementary Materials for The mutagenic chain reaction: A method for converting heterozygous to homozygous mutations Valentino M. Gantz* and Ethan
www.sciencemag.org/cgi/content/full/science.aaa5945/dc1 Supplementary Materials for The mutagenic chain reaction: A method for converting heterozygous to homozygous mutations Valentino M. Gantz* and Ethan
XactEdit Cas9 Nuclease with NLS User Manual
 XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
Easi CRISPR for conditional and insertional alleles
 Easi CRISPR for conditional and insertional alleles C.B Gurumurthy, University Of Nebraska Medical Center Omaha, NE cgurumurthy@unmc.edu Types of Genome edits Gene disruption/inactivation Types of Genome
Easi CRISPR for conditional and insertional alleles C.B Gurumurthy, University Of Nebraska Medical Center Omaha, NE cgurumurthy@unmc.edu Types of Genome edits Gene disruption/inactivation Types of Genome
CRISPR Ribonucleoprotein (RNP) User Manual
 CRISPR Ribonucleoprotein (RNP) User Manual Description This user manual describes how to use GenScript s CRISPR Ribonucleoprotein (RNP) products for targeted genome editing. The RNP system is comprised
CRISPR Ribonucleoprotein (RNP) User Manual Description This user manual describes how to use GenScript s CRISPR Ribonucleoprotein (RNP) products for targeted genome editing. The RNP system is comprised
A Guide to CRISPR/Cas9
 Genome editing and beyond freepik A Guide to CRISPR/Cas9 The latest advance in genomic DNA editing is the Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)/Cas9 system. This simple-touse
Genome editing and beyond freepik A Guide to CRISPR/Cas9 The latest advance in genomic DNA editing is the Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)/Cas9 system. This simple-touse
Chapter 6 - Molecular Genetic Techniques
 Chapter 6 - Molecular Genetic Techniques Two objects of molecular & genetic technologies For analysis For generation Molecular genetic technologies! For analysis DNA gel electrophoresis Southern blotting
Chapter 6 - Molecular Genetic Techniques Two objects of molecular & genetic technologies For analysis For generation Molecular genetic technologies! For analysis DNA gel electrophoresis Southern blotting
Boosting CRISPR/Cas9 multiplex editing capability with the. endogenous trna processing system
 Supporting Information Boosting CRISPR/Cas9 multiplex editing capability with the endogenous trna processing system Kabin Xie, Bastian Minkenberg and Yinong Yang Department of Plant Pathology and Environmental
Supporting Information Boosting CRISPR/Cas9 multiplex editing capability with the endogenous trna processing system Kabin Xie, Bastian Minkenberg and Yinong Yang Department of Plant Pathology and Environmental
Data Sheet Quick PCR Cloning Kit
 Data Sheet Quick PCR Cloning Kit 6044 Cornerstone Ct. West, Ste. E DESCRIPTION: The Quick PCR Cloning Kit is a simple and highly efficient method to insert any gene or DNA fragment into a vector, without
Data Sheet Quick PCR Cloning Kit 6044 Cornerstone Ct. West, Ste. E DESCRIPTION: The Quick PCR Cloning Kit is a simple and highly efficient method to insert any gene or DNA fragment into a vector, without
CELLTECHGEN For Research Only. Construction of sgrna expression vector for Lenti-virus system (Example: Lenti-U6 sgrna-ef1 -Puro vector)
 Construction of sgrna expression vector for Lenti-virus system (Example: Lenti-U6 sgrna-ef1 -Puro vector) Catalog number: CTG-CAS9-11 Introduction The vector Lenti-U6 sgrna-ef1 -Puro is designed for expression
Construction of sgrna expression vector for Lenti-virus system (Example: Lenti-U6 sgrna-ef1 -Puro vector) Catalog number: CTG-CAS9-11 Introduction The vector Lenti-U6 sgrna-ef1 -Puro is designed for expression
Supplementary Information
 Supplementary Information Vidigal and Ventura a wt locus 5 region 3 region CCTCTGCCACTGCGAGGGCGTCCAATGGTGCTTG(...)AACAGGTGGAATATCCCTACTCTA predicted deletion clone 1 clone 2 clone 3 CCTCTGCCACTGCGAGGGCGTC-AGGTGGAATATCCCTACTCTA
Supplementary Information Vidigal and Ventura a wt locus 5 region 3 region CCTCTGCCACTGCGAGGGCGTCCAATGGTGCTTG(...)AACAGGTGGAATATCCCTACTCTA predicted deletion clone 1 clone 2 clone 3 CCTCTGCCACTGCGAGGGCGTC-AGGTGGAATATCCCTACTCTA
Supplementary Information
 Supplementary Information Super-resolution imaging of fluorescently labeled, endogenous RNA Polymerase II in living cells with CRISPR/Cas9-mediated gene editing Won-Ki Cho 1, Namrata Jayanth 1, Susan Mullen
Supplementary Information Super-resolution imaging of fluorescently labeled, endogenous RNA Polymerase II in living cells with CRISPR/Cas9-mediated gene editing Won-Ki Cho 1, Namrata Jayanth 1, Susan Mullen
Supplementary data. sienigma. F-Enigma F-EnigmaSM. a-p53
 Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Supporting Information
 Supporting Information Park et al. 10.1073/pnas.1410555111 5 -TCAAGTCCATCTACATGGCC-3 5 -CAGCTGCCCGGCTACTACTA-3 5 -TGCAGCTGCCCGGCTACTAC-3 5 -AAGCTGGACATCACCTCCCA-3 5 -TGACAGGAACACCTACAAGT-3 5 -AAGGCACCTTTCTGTCTCCA-3
Supporting Information Park et al. 10.1073/pnas.1410555111 5 -TCAAGTCCATCTACATGGCC-3 5 -CAGCTGCCCGGCTACTACTA-3 5 -TGCAGCTGCCCGGCTACTAC-3 5 -AAGCTGGACATCACCTCCCA-3 5 -TGACAGGAACACCTACAAGT-3 5 -AAGGCACCTTTCTGTCTCCA-3
Reading Lecture 8: Lecture 9: Lecture 8. DNA Libraries. Definition Types Construction
 Lecture 8 Reading Lecture 8: 96-110 Lecture 9: 111-120 DNA Libraries Definition Types Construction 142 DNA Libraries A DNA library is a collection of clones of genomic fragments or cdnas from a certain
Lecture 8 Reading Lecture 8: 96-110 Lecture 9: 111-120 DNA Libraries Definition Types Construction 142 DNA Libraries A DNA library is a collection of clones of genomic fragments or cdnas from a certain
TRANSGENIC ANIMALS. -transient transfection of cells -stable transfection of cells. - Two methods to produce transgenic animals:
 TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
Supplementary information, Figure S1
 Supplementary information, Figure S1 (A) Schematic diagram of the sgrna and hspcas9 expression cassettes in a single binary vector designed for Agrobacterium-mediated stable transformation of Arabidopsis
Supplementary information, Figure S1 (A) Schematic diagram of the sgrna and hspcas9 expression cassettes in a single binary vector designed for Agrobacterium-mediated stable transformation of Arabidopsis
Using CRISPR for genetic alteration
 Using CRISPR for genetic alteration Joffrey Mianné. j.mianne@har.mrc.ac.uk Mary Lyon Centre, MRC Harwell. CRISPR/Cas origins Origin of the CRISPR/Cas system: Clustered-Regularly Interspaced Short Palindromic
Using CRISPR for genetic alteration Joffrey Mianné. j.mianne@har.mrc.ac.uk Mary Lyon Centre, MRC Harwell. CRISPR/Cas origins Origin of the CRISPR/Cas system: Clustered-Regularly Interspaced Short Palindromic
Supplementary Information
 Supplementary Information Generation of inheritable and transgene clean targeted genome-modified rice in later generations using the CRISPR/Cas9 system. Rong-Fang Xu, Hao Li, Rui-Ying Qin, Juan Li, Chun-Hong
Supplementary Information Generation of inheritable and transgene clean targeted genome-modified rice in later generations using the CRISPR/Cas9 system. Rong-Fang Xu, Hao Li, Rui-Ying Qin, Juan Li, Chun-Hong
Supplemental information Precise A T to G C base editing in the rice genome
 Supplemental information Precise A T to G C base editing in the rice genome Kai Hua, Xiaoping Tao, Fengtong Yuan, Dong Wang, Jian-Kang Zhu Contents Supplemental Figure 1. TA cloning results of two base
Supplemental information Precise A T to G C base editing in the rice genome Kai Hua, Xiaoping Tao, Fengtong Yuan, Dong Wang, Jian-Kang Zhu Contents Supplemental Figure 1. TA cloning results of two base
BS 50 Genetics and Genomics Week of Oct 24
 BS 50 Genetics and Genomics Week of Oct 24 Additional Practice Problems for Section Question 1: The following table contains a list of statements that apply to replication, transcription, both, or neither.
BS 50 Genetics and Genomics Week of Oct 24 Additional Practice Problems for Section Question 1: The following table contains a list of statements that apply to replication, transcription, both, or neither.
Supplementary Materials. China
 Supplementary Materials An Efficient Genotyping Method for Genome-modified Animals and Human Cells Generated with CRISPR/Cas9 System Xiaoxiao Zhu 1,2 *, Yajie Xu 1 *, Shanshan Yu 1 *, Lu Lu 1,2, Mingqin
Supplementary Materials An Efficient Genotyping Method for Genome-modified Animals and Human Cells Generated with CRISPR/Cas9 System Xiaoxiao Zhu 1,2 *, Yajie Xu 1 *, Shanshan Yu 1 *, Lu Lu 1,2, Mingqin
Enzyme that uses RNA as a template to synthesize a complementary DNA
 Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Comparison of two or more protein or DNA sequence to ascertain similarities in sequences. If two genes have
Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Comparison of two or more protein or DNA sequence to ascertain similarities in sequences. If two genes have
B. Incorrect! Ligation is also a necessary step for cloning.
 Genetics - Problem Drill 15: The Techniques in Molecular Genetics No. 1 of 10 1. Which of the following is not part of the normal process of cloning recombinant DNA in bacteria? (A) Restriction endonuclease
Genetics - Problem Drill 15: The Techniques in Molecular Genetics No. 1 of 10 1. Which of the following is not part of the normal process of cloning recombinant DNA in bacteria? (A) Restriction endonuclease
(i) A trp1 mutant cell took up a plasmid containing the wild type TRP1 gene, which allowed that cell to multiply and form a colony
 1. S. pombe is a distant relative of baker s yeast (which you used in quiz section). Wild type S. pombe can grow on plates lacking tryptophan (-trp plates). A mutant has been isolated that cannot grow
1. S. pombe is a distant relative of baker s yeast (which you used in quiz section). Wild type S. pombe can grow on plates lacking tryptophan (-trp plates). A mutant has been isolated that cannot grow
Molecular Cell Biology - Problem Drill 11: Recombinant DNA
 Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
EnGen Mutation Detection Kit
 DNA MODIFYING ENZYMES EnGen Mutation Detection Kit Instruction Manual NEB #E3321S 25 reactions Version 2.0 6/18 be INSPIRED drive DISCOVERY stay GENUINE This product is intended for research purposes only.
DNA MODIFYING ENZYMES EnGen Mutation Detection Kit Instruction Manual NEB #E3321S 25 reactions Version 2.0 6/18 be INSPIRED drive DISCOVERY stay GENUINE This product is intended for research purposes only.
Supplemental Information
 Supplemental Information ATP-dependent unwinding of U4/U6 snrnas by the Brr2 helicase requires the C-terminus of Prp8 Corina Maeder 1,3, Alan K. Kutach 1,2,3, and Christine Guthrie 1 1 Department of Biochemistry
Supplemental Information ATP-dependent unwinding of U4/U6 snrnas by the Brr2 helicase requires the C-terminus of Prp8 Corina Maeder 1,3, Alan K. Kutach 1,2,3, and Christine Guthrie 1 1 Department of Biochemistry
Human Molecular Genetics Assignment 3 (Week 3)
 Human Molecular Genetics Assignment 3 (Week 3) Q1. Which one of the following is an effect of a genetic mutation? a. Prevent the synthesis of a normal protein. b. Alters the function of the resulting protein
Human Molecular Genetics Assignment 3 (Week 3) Q1. Which one of the following is an effect of a genetic mutation? a. Prevent the synthesis of a normal protein. b. Alters the function of the resulting protein
High-throughput genotyping of CRISPR/Cas9-mediated mutants using fluorescent
 High-throughput genotyping of CRISPR/Cas9-mediated mutants using fluorescent PCR-capillary gel electrophoresis Muhammad Khairul RAMLEE, Tingdong YAN, Alice M. S. CHEUNG, Charles CHUAH, Shang LI Figure
High-throughput genotyping of CRISPR/Cas9-mediated mutants using fluorescent PCR-capillary gel electrophoresis Muhammad Khairul RAMLEE, Tingdong YAN, Alice M. S. CHEUNG, Charles CHUAH, Shang LI Figure
Genome-wide genetic screening with chemically-mutagenized haploid embryonic stem cells
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Supplementary Information Genome-wide genetic screening with chemically-mutagenized haploid embryonic stem cells Josep V. Forment 1,2, Mareike Herzog
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Supplementary Information Genome-wide genetic screening with chemically-mutagenized haploid embryonic stem cells Josep V. Forment 1,2, Mareike Herzog
HE Swift Cloning Kit
 HE Swift Cloning Kit For high-efficient cloning of PCR products either blunt or sticky-end Kit Contents Contents VTT-BB05 phe Vector (35 ng/µl) 20 µl T4 DNA Ligase (3 U/µl) 20 µl 2 Reaction Buffer 100
HE Swift Cloning Kit For high-efficient cloning of PCR products either blunt or sticky-end Kit Contents Contents VTT-BB05 phe Vector (35 ng/µl) 20 µl T4 DNA Ligase (3 U/µl) 20 µl 2 Reaction Buffer 100
7.012 Problem Set 5. Question 1
 Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Amplicons, Heteroduplexes and Enzymes - Proper Processing Elevates Detection of CRISPR Gene Editing Events
 Amplicons, Heteroduplexes and Enzymes - Proper Processing Elevates Detection of CRISPR Gene Editing Events Steve Siembieda, MS MBA VP Commercialization ABRF Conference February 2015 What Is CRISPR? Clustered
Amplicons, Heteroduplexes and Enzymes - Proper Processing Elevates Detection of CRISPR Gene Editing Events Steve Siembieda, MS MBA VP Commercialization ABRF Conference February 2015 What Is CRISPR? Clustered
ksierzputowska.com Research Title: Using novel TALEN technology to engineer precise mutations in the genome of D. melanogaster
 Research Title: Using novel TALEN technology to engineer precise mutations in the genome of D. melanogaster Research plan: Specific aims: 1. To successfully engineer transgenic Drosophila expressing TALENs
Research Title: Using novel TALEN technology to engineer precise mutations in the genome of D. melanogaster Research plan: Specific aims: 1. To successfully engineer transgenic Drosophila expressing TALENs
A) (5 points) As the starting step isolate genomic DNA from
 GS Final Exam Spring 00 NAME. bub ts is a recessive temperature sensitive mutation in yeast. At º C bub ts cells grow normally, but at º C they die. Use the information below to clone the wild-type BUB
GS Final Exam Spring 00 NAME. bub ts is a recessive temperature sensitive mutation in yeast. At º C bub ts cells grow normally, but at º C they die. Use the information below to clone the wild-type BUB
Nature Biotechnology: doi: /nbt Supplementary Figure 1. In vitro validation of OTC sgrnas and donor template.
 Supplementary Figure 1 In vitro validation of OTC sgrnas and donor template. (a) In vitro validation of sgrnas targeted to OTC in the MC57G mouse cell line by transient transfection followed by 4-day puromycin
Supplementary Figure 1 In vitro validation of OTC sgrnas and donor template. (a) In vitro validation of sgrnas targeted to OTC in the MC57G mouse cell line by transient transfection followed by 4-day puromycin
Supplementary Figure 1 Activities of ABEs using extended sgrnas in HEK293T cells.
 Supplementary Figure 1 Activities of ABEs using extended sgrnas in HEK293T cells. Base editing efficiencies of ABEs with extended sgrnas at Site 18 (a), Site 19 (b), the HBB-E2 site (c), and the HBB-E3
Supplementary Figure 1 Activities of ABEs using extended sgrnas in HEK293T cells. Base editing efficiencies of ABEs with extended sgrnas at Site 18 (a), Site 19 (b), the HBB-E2 site (c), and the HBB-E3
CRISPR/Cas9 Mouse Production
 CRISPR/Cas9 Mouse Production Emory Transgenic and Gene Targeting Core http://cores.emory.edu/tmc Tamara Caspary, Ph.D. Scientific Director Teresa Quackenbush --- Lab Operations and Communications Coordinator
CRISPR/Cas9 Mouse Production Emory Transgenic and Gene Targeting Core http://cores.emory.edu/tmc Tamara Caspary, Ph.D. Scientific Director Teresa Quackenbush --- Lab Operations and Communications Coordinator
Targeted Gene Mutation in Rice Using a CRISPR-Cas9 System Kabin Xie 1, Bastian Minkenberg 2 and Yinong Yang 2*
 Targeted Gene Mutation in Rice Using a CRISPR-Cas9 System Kabin Xie 1, Bastian Minkenberg 2 and Yinong Yang 2* 1 Department of Plant Pathology and Environmental Microbiology, Pennsylvania State University,
Targeted Gene Mutation in Rice Using a CRISPR-Cas9 System Kabin Xie 1, Bastian Minkenberg 2 and Yinong Yang 2* 1 Department of Plant Pathology and Environmental Microbiology, Pennsylvania State University,
Lentiviral CRISPR guild RNA Cloning Kit for constructing CRISPR targeting grna lentivectors
 Lentiviral CRISPR guild RNA Cloning Kit for constructing CRISPR targeting grna lentivectors Cat# Product Name Amount Application grna-h1-gb grna-h1-gp grna-h1-rb grna-h1-rp grna-h1-puro grna-h1-bsd grna-u6-gb
Lentiviral CRISPR guild RNA Cloning Kit for constructing CRISPR targeting grna lentivectors Cat# Product Name Amount Application grna-h1-gb grna-h1-gp grna-h1-rb grna-h1-rp grna-h1-puro grna-h1-bsd grna-u6-gb
Inhibition of HSV-1 Replication by Gene Editing Strategy. Running Title: Targeting HSV-1 Infection by CRISPR/Cas9
 SUPPLEMENTARY MATERIALS 2/4/16 Inhibition of HSV-1 Replication by Gene Editing Strategy Running Title: Targeting HSV-1 Infection by CRISPR/Cas9 Pamela C. Roehm 1,2,3*, Masoud Shekarabi 1, Hassen Wollebo
SUPPLEMENTARY MATERIALS 2/4/16 Inhibition of HSV-1 Replication by Gene Editing Strategy Running Title: Targeting HSV-1 Infection by CRISPR/Cas9 Pamela C. Roehm 1,2,3*, Masoud Shekarabi 1, Hassen Wollebo
Ligation Independent Cloning (LIC) Procedure
 Ligation Independent Cloning (LIC) Procedure Ligation Independent Cloning (LIC) LIC cloning allows insertion of DNA fragments without using restriction enzymes into specific vectors containing engineered
Ligation Independent Cloning (LIC) Procedure Ligation Independent Cloning (LIC) LIC cloning allows insertion of DNA fragments without using restriction enzymes into specific vectors containing engineered
i-stop codon positions in the mcherry gene
 Supplementary Figure 1 i-stop codon positions in the mcherry gene The grnas (green) that can potentially generate stop codons from Trp (63 th and 98 th aa, upper panel) and Gln (47 th and 114 th aa, bottom
Supplementary Figure 1 i-stop codon positions in the mcherry gene The grnas (green) that can potentially generate stop codons from Trp (63 th and 98 th aa, upper panel) and Gln (47 th and 114 th aa, bottom
File S1. Detailed protocol for CRISPR/Cas9 mediated genome editing with dual marker cassettes
 File S1 Detailed protocol for CRISPR/Cas9 mediated genome editing with dual marker cassettes A) Preparing sgrna vectors We ve modified the strategy to create new sgrna vectors starting with the klp 12
File S1 Detailed protocol for CRISPR/Cas9 mediated genome editing with dual marker cassettes A) Preparing sgrna vectors We ve modified the strategy to create new sgrna vectors starting with the klp 12
Supplementary Information
 Single day construction of multi-gene circuits with 3G assembly Andrew D. Halleran 1, Anandh Swaminathan 2, and Richard M. Murray 1, 2 1. Bioengineering, California Institute of Technology, Pasadena, CA.
Single day construction of multi-gene circuits with 3G assembly Andrew D. Halleran 1, Anandh Swaminathan 2, and Richard M. Murray 1, 2 1. Bioengineering, California Institute of Technology, Pasadena, CA.
Molecular Genetics Techniques. BIT 220 Chapter 20
 Molecular Genetics Techniques BIT 220 Chapter 20 What is Cloning? Recombinant DNA technologies 1. Producing Recombinant DNA molecule Incorporate gene of interest into plasmid (cloning vector) 2. Recombinant
Molecular Genetics Techniques BIT 220 Chapter 20 What is Cloning? Recombinant DNA technologies 1. Producing Recombinant DNA molecule Incorporate gene of interest into plasmid (cloning vector) 2. Recombinant
Genome Engineering with ZFNs, TALENs and CRISPR/Cas9
 Genome Engineering with ZFNs, TALENs and CRISPR/Cas9 Designer Endonucleases ZFNs (zinc finger nucleases), TALENs (transcription activator-like effector nucleases) and CRISPR/Cas9 (clustered regularly interspaced
Genome Engineering with ZFNs, TALENs and CRISPR/Cas9 Designer Endonucleases ZFNs (zinc finger nucleases), TALENs (transcription activator-like effector nucleases) and CRISPR/Cas9 (clustered regularly interspaced
Supplemental Protocols Gagnon et al., 2014
 Last updated April 15th 2014 There may be a more up-to-date version of this protocol on our website http://labs.mcb.harvard.edu/schier/index.html Protocol Page Flowchart for Cas9-mediated mutagenesis 1
Last updated April 15th 2014 There may be a more up-to-date version of this protocol on our website http://labs.mcb.harvard.edu/schier/index.html Protocol Page Flowchart for Cas9-mediated mutagenesis 1
A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish
 Developmental Cell Supplemental Information A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish Julien Ablain, Ellen M. Durand, Song Yang, Yi Zhou, and Leonard I. Zon % larvae
Developmental Cell Supplemental Information A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish Julien Ablain, Ellen M. Durand, Song Yang, Yi Zhou, and Leonard I. Zon % larvae
Analysis of gene function
 Genome 371, 22 February 2010, Lecture 12 Analysis of gene function Gene knockouts PHASE TWO: INTERPRETATION I THINK I FOUND A CORNER PIECE. 3 BILLION PIECES Analysis of a disease gene Gene knockout or
Genome 371, 22 February 2010, Lecture 12 Analysis of gene function Gene knockouts PHASE TWO: INTERPRETATION I THINK I FOUND A CORNER PIECE. 3 BILLION PIECES Analysis of a disease gene Gene knockout or
Supplementary Figure 1 An overview of pirna biogenesis during fetal mouse reprogramming. (a) (b)
 Supplementary Figure 1 An overview of pirna biogenesis during fetal mouse reprogramming. (a) A schematic overview of the production and amplification of a single pirna from a transposon transcript. The
Supplementary Figure 1 An overview of pirna biogenesis during fetal mouse reprogramming. (a) A schematic overview of the production and amplification of a single pirna from a transposon transcript. The
NZYGene Synthesis kit
 Kit components Component Concentration Amount NZYGene Synthesis kit Catalogue number: MB33901, 10 reactions GS DNA Polymerase 1U/ μl 30 μl Reaction Buffer for GS DNA Polymerase 10 150 μl dntp mix 2 mm
Kit components Component Concentration Amount NZYGene Synthesis kit Catalogue number: MB33901, 10 reactions GS DNA Polymerase 1U/ μl 30 μl Reaction Buffer for GS DNA Polymerase 10 150 μl dntp mix 2 mm
GenBuilder TM Plus Cloning Kit User Manual
 GenBuilder TM Plus Cloning Kit User Manual Cat. No. L00744 Version 11242017 Ⅰ. Introduction... 2 I.1 Product Information... 2 I.2 Kit Contents and Storage... 2 I.3 GenBuilder Cloning Kit Workflow... 2
GenBuilder TM Plus Cloning Kit User Manual Cat. No. L00744 Version 11242017 Ⅰ. Introduction... 2 I.1 Product Information... 2 I.2 Kit Contents and Storage... 2 I.3 GenBuilder Cloning Kit Workflow... 2
GenBuilder TM Plus Cloning Kit User Manual
 GenBuilder TM Plus Cloning Kit User Manual Cat.no L00744 Version 11242017 Ⅰ. Introduction... 2 I.1 Product Information... 2 I.2 Kit Contents and Storage... 2 I.3 GenBuilder Cloning Kit Workflow... 2 Ⅱ.
GenBuilder TM Plus Cloning Kit User Manual Cat.no L00744 Version 11242017 Ⅰ. Introduction... 2 I.1 Product Information... 2 I.2 Kit Contents and Storage... 2 I.3 GenBuilder Cloning Kit Workflow... 2 Ⅱ.
DESIGNER GENES - BIOTECHNOLOGY
 DESIGNER GENES - BIOTECHNOLOGY Technology to manipulate DNA techniques often called genetic engineering or Recombinant DNA Technology-Technology used to manipulate DNA Procedures often called genetic engineering
DESIGNER GENES - BIOTECHNOLOGY Technology to manipulate DNA techniques often called genetic engineering or Recombinant DNA Technology-Technology used to manipulate DNA Procedures often called genetic engineering
XXII DNA cloning and sequencing. Outline
 XXII DNA cloning and sequencing 1) Deriving DNA for cloning Outline 2) Vectors; forming recombinant DNA; cloning DNA; and screening for clones containing recombinant DNA [replica plating and autoradiography;
XXII DNA cloning and sequencing 1) Deriving DNA for cloning Outline 2) Vectors; forming recombinant DNA; cloning DNA; and screening for clones containing recombinant DNA [replica plating and autoradiography;
Supplementary Figure 1. Nature Structural & Molecular Biology: doi: /nsmb.3494
 Supplementary Figure 1 Pol structure-function analysis (a) Inactivating polymerase and helicase mutations do not alter the stability of Pol. Flag epitopes were introduced using CRISPR/Cas9 gene targeting
Supplementary Figure 1 Pol structure-function analysis (a) Inactivating polymerase and helicase mutations do not alter the stability of Pol. Flag epitopes were introduced using CRISPR/Cas9 gene targeting
CRISPR RNA-guided activation of endogenous human genes
 CRISPR RNA-guided activation of endogenous human genes Morgan L Maeder, Samantha J Linder, Vincent M Cascio, Yanfang Fu, Quan H Ho, J Keith Joung Supplementary Figure 1 Comparison of VEGF activation induced
CRISPR RNA-guided activation of endogenous human genes Morgan L Maeder, Samantha J Linder, Vincent M Cascio, Yanfang Fu, Quan H Ho, J Keith Joung Supplementary Figure 1 Comparison of VEGF activation induced
MID-TERM EXAMINATION
 PLNT3140 INTRODUCTORY CYTOGENETICS MID-TERM EXAMINATION 1 p.m. to 2:15 p.m. Thursday, October 18, 2012 Answer any combination of questions totalling to exactly 100 points. If you answer questions totalling
PLNT3140 INTRODUCTORY CYTOGENETICS MID-TERM EXAMINATION 1 p.m. to 2:15 p.m. Thursday, October 18, 2012 Answer any combination of questions totalling to exactly 100 points. If you answer questions totalling
BIOLOGY - CLUTCH CH.20 - BIOTECHNOLOGY.
 !! www.clutchprep.com CONCEPT: DNA CLONING DNA cloning is a technique that inserts a foreign gene into a living host to replicate the gene and produce gene products. Transformation the process by which
!! www.clutchprep.com CONCEPT: DNA CLONING DNA cloning is a technique that inserts a foreign gene into a living host to replicate the gene and produce gene products. Transformation the process by which
Combinatorial Evolution of Enzymes and Synthetic pathways Using
 Supplementary data Combinatorial Evolution of Enzymes and Synthetic pathways Using One-Step PCR Peng Jin,, Zhen Kang,,, *, Junli Zhang,, Linpei Zhang,, Guocheng Du,, and Jian Chen, The Key Laboratory of
Supplementary data Combinatorial Evolution of Enzymes and Synthetic pathways Using One-Step PCR Peng Jin,, Zhen Kang,,, *, Junli Zhang,, Linpei Zhang,, Guocheng Du,, and Jian Chen, The Key Laboratory of
GenBuilder TM Cloning Kit User Manual
 GenBuilder TM Cloning Kit User Manual Cat.no L00701 Version 11242017 Ⅰ. Introduction... 2 I.1 Product Information... 2 I.2 Kit Contents and Storage... 2 I.3 GenBuilder Cloning Kit Workflow... 2 Ⅱ. DNA
GenBuilder TM Cloning Kit User Manual Cat.no L00701 Version 11242017 Ⅰ. Introduction... 2 I.1 Product Information... 2 I.2 Kit Contents and Storage... 2 I.3 GenBuilder Cloning Kit Workflow... 2 Ⅱ. DNA
Recombinant DNA Technology
 Recombinant DNA Technology Common General Cloning Strategy Target DNA from donor organism extracted, cut with restriction endonuclease and ligated into a cloning vector cut with compatible restriction
Recombinant DNA Technology Common General Cloning Strategy Target DNA from donor organism extracted, cut with restriction endonuclease and ligated into a cloning vector cut with compatible restriction
Introduction to CRISPR/Cas9 Background DNA Cleavage and Repair (NHEJ and HDR) Alternative Cas9 Variants Delivery of Cas9 and sgrna Library Products
 Introduction to CRISPR/Cas9 Background DNA Cleavage and Repair (NHEJ and HDR) Alternative Cas9 Variants Delivery of Cas9 and sgrna Library Products which one is right for you? CRISPR Workflow abm s Toolbox
Introduction to CRISPR/Cas9 Background DNA Cleavage and Repair (NHEJ and HDR) Alternative Cas9 Variants Delivery of Cas9 and sgrna Library Products which one is right for you? CRISPR Workflow abm s Toolbox
User Instructions:Transfection-ready CRISPR/Cas9 Reagents. Target DNA. NHEJ repair pathway. Nucleotide deletion. Nucleotide insertion Gene disruption
 User Instructions:Transfection-ready CRISPR/Cas9 Reagents Background Introduction to CRISPR/Cas9 genome editing In bacteria and archaea, clustered regularly interspaced short palindromic repeats (CRISPR)
User Instructions:Transfection-ready CRISPR/Cas9 Reagents Background Introduction to CRISPR/Cas9 genome editing In bacteria and archaea, clustered regularly interspaced short palindromic repeats (CRISPR)
GENOME 371, Problem Set 6
 GENOME 371, Problem Set 6 1. S. pombe is a distant relative of baker s yeast (which you used in quiz section). Wild type S. pombe can grow on plates lacking tryptophan (-trp plates). A mutant has been
GENOME 371, Problem Set 6 1. S. pombe is a distant relative of baker s yeast (which you used in quiz section). Wild type S. pombe can grow on plates lacking tryptophan (-trp plates). A mutant has been
2014 Pearson Education, Inc. CH 8: Recombinant DNA Technology
 CH 8: Recombinant DNA Technology Biotechnology the use of microorganisms to make practical products Recombinant DNA = DNA from 2 different sources What is Recombinant DNA Technology? modifying genomes
CH 8: Recombinant DNA Technology Biotechnology the use of microorganisms to make practical products Recombinant DNA = DNA from 2 different sources What is Recombinant DNA Technology? modifying genomes
GENETICS EXAM 3 FALL a) is a technique that allows you to separate nucleic acids (DNA or RNA) by size.
 Student Name: All questions are worth 5 pts. each. GENETICS EXAM 3 FALL 2004 1. a) is a technique that allows you to separate nucleic acids (DNA or RNA) by size. b) Name one of the materials (of the two
Student Name: All questions are worth 5 pts. each. GENETICS EXAM 3 FALL 2004 1. a) is a technique that allows you to separate nucleic acids (DNA or RNA) by size. b) Name one of the materials (of the two
Module Code: BIO00007C
 Examination Candidate Number: Desk Number: BSc and MSc Degree Examinations 2018-9 Department : BIOLOGY Title of Exam: Genetics Time Allowed: 1 Hour 30 Minutes Marking Scheme: Total marks available for
Examination Candidate Number: Desk Number: BSc and MSc Degree Examinations 2018-9 Department : BIOLOGY Title of Exam: Genetics Time Allowed: 1 Hour 30 Minutes Marking Scheme: Total marks available for
The Biotechnology Toolbox
 Chapter 15 The Biotechnology Toolbox Cutting and Pasting DNA Cutting DNA Restriction endonuclease or restriction enzymes Cellular protection mechanism for infected foreign DNA Recognition and cutting specific
Chapter 15 The Biotechnology Toolbox Cutting and Pasting DNA Cutting DNA Restriction endonuclease or restriction enzymes Cellular protection mechanism for infected foreign DNA Recognition and cutting specific
Supplementary Figure 1. Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings.
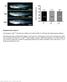 Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Biology 105: Introduction to Genetics PRACTICE FINAL EXAM Part I: Definitions. Homology: Reverse transcriptase. Allostery: cdna library
 Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Reverse transcriptase Allostery: cdna library Transformation Part II Short Answer 1. Describe the reasons for
Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Reverse transcriptase Allostery: cdna library Transformation Part II Short Answer 1. Describe the reasons for
PlantDirect TM Multiplex PCR System
 PlantDirect TM Multiplex PCR System Technical Manual No. 0178 Version 10112010 I Description.. 1 II Applications 2 III Key Features.. 3 IV Shipping and Storage. 3 V Simplified Procedures. 3 VI Detailed
PlantDirect TM Multiplex PCR System Technical Manual No. 0178 Version 10112010 I Description.. 1 II Applications 2 III Key Features.. 3 IV Shipping and Storage. 3 V Simplified Procedures. 3 VI Detailed
The Polymerase Chain Reaction. Chapter 6: Background
 The Polymerase Chain Reaction Chapter 6: Background Invention of PCR Kary Mullis Mile marker 46.58 in April of 1983 Pulled off the road and outlined a way to conduct DNA replication in a tube Worked for
The Polymerase Chain Reaction Chapter 6: Background Invention of PCR Kary Mullis Mile marker 46.58 in April of 1983 Pulled off the road and outlined a way to conduct DNA replication in a tube Worked for
SUPPORTING INFORMATION. A cleavage-responsive stem-loop hairpin for assaying guide RNA activity
 SUPPORTING INFORMATION A cleavage-responsive stem-loop hairpin for assaying guide RNA activity Tara R. deboer 1, Noreen Wauford 1, Jing-Yi Chung, Miguel Salvador Torres Perez, and Niren Murthy* University
SUPPORTING INFORMATION A cleavage-responsive stem-loop hairpin for assaying guide RNA activity Tara R. deboer 1, Noreen Wauford 1, Jing-Yi Chung, Miguel Salvador Torres Perez, and Niren Murthy* University
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION High-efficiency TALEN-based gene editing produces disease-resistance rice Ting Li 1, Bo Liu 1, Martin H. Spalding 1, Donald P. Weeks 2, Bing Yang 1 * 1 Department of Genetics,
SUPPLEMENTARY INFORMATION High-efficiency TALEN-based gene editing produces disease-resistance rice Ting Li 1, Bo Liu 1, Martin H. Spalding 1, Donald P. Weeks 2, Bing Yang 1 * 1 Department of Genetics,
Authors: Vivek Sharma and Ram Kunwar
 Molecular markers types and applications A genetic marker is a gene or known DNA sequence on a chromosome that can be used to identify individuals or species. Why we need Molecular Markers There will be
Molecular markers types and applications A genetic marker is a gene or known DNA sequence on a chromosome that can be used to identify individuals or species. Why we need Molecular Markers There will be
Ready_to_use Fast Seamless Cloning Kit. User Manual
 For general laboratory use. Not for use in diagnostic procedures. FOR IN VITRO USE ONLY. Ready_to_use Fast Seamless Cloning Kit User Manual 1 / 6 Tel: 021-58975266 Fax: 021-50800270 Email:tech@dogene.com
For general laboratory use. Not for use in diagnostic procedures. FOR IN VITRO USE ONLY. Ready_to_use Fast Seamless Cloning Kit User Manual 1 / 6 Tel: 021-58975266 Fax: 021-50800270 Email:tech@dogene.com
