JOURNAL OF NEUROCHEMISTRY doi: /jnc.12243
|
|
|
- Hilary Manning
- 5 years ago
- Views:
Transcription
1 JOURNAL OF NEUROCHEMISTRY doi: /jnc.12243, *Department of Nanobiophotonics, Max Planck Institute for Biophysical Chemistry, G ottingen, Germany Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, UK Laboratory of Molecular Neurobiology, Institute of Biomedical Research (BIOMED) UCA CONICET, Catholic University of Argentina, Buenos Aires, Argentina Abstract Recent developments in fluorescence far-field microscopy such as STED microscopy have accomplished observation of the living cell with a spatial resolution far below the diffraction limit. Here, we briefly review the current approaches to superresolution optical microscopy and present the implementation of STED microscopy for novel insights into live cell mechanisms, with a focus on neurobiology and plasma membrane dynamics. Keywords: fluorescence imaging, membrane dynamics, STED nanoscopy. J. Neurochem. (2013) 126, Microscopy has revolutionized our understanding of life and matter, since it allows the observation of molecular organization in complex biological systems. Among all the different microscopy techniques, optical microscopy is one of the least invasive approaches: the use of light hardly influences the system under study. Optical microscopy of the living cell is usually applied in the far-field, where the use of a lens-based system and focused light allows the observation micrometers to millimeters away from the given optical element(s), preserving the non-invasiveness and the ability to observe deep inside living cells or tissues. Further, when combined with the fluorescence readout (e.g., fluorescentlabeled molecules), far-field fluorescence microscopy achieves the specific and highly sensitive detection of cellular constituents and thus permits the disclosure of molecular distributions and dynamics in the living cell. However, a number of challenging issues in biology lie beyond the reach of conventional far-field optical microscopy, especially because of its limited spatial resolution. When using visible and focused light, light diffraction prevents the discernment of alike objects closer together than approximately 200 nm (Abbe 1873). A remedy to this physical limit is the reversible inhibition of fluorescence, ensuring that the measured signal stems from a region of the sample that is much smaller than 200 nm (Hell 2007). Starting in 1994 (Hell and Wichmann 1994) different farfield fluorescence super-resolution microscopy or nanoscopy approaches have emerged, all capable of achieving cellular imaging with in principle unlimited spatial resolution. Examples include optical Stimulated Emission Depletion (STED) (Hell and Wichmann 1994; Klar et al. 2000), Reversible Saturable Optical (Fluorescence) Transitions (RESOLFT) (Hell et al. 2003; Hofmann et al. 2005; Grotjohann et al. 2011) Ground State Depletion (GSD) (Hell and Kroug 1995; Bretschneider et al. 2007; Han et al. 2010) nanoscopy, Stochastic Optical Reconstruction Microscopy (STORM) (Rust et al. 2006), and (fluorescence) Received December 14, 2012; revised manuscript received February 18, 2013; accepted March 13, Address correspondence and reprint requests to Christian Eggeling, Department of Nanobiophotonics, Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, G ottingen, Germany. ceggeli@gwdg.de Abbreviations used: cltp, chemical long-term potentiation; dstorm, direct STORM; FCS, fluorescence correlation spectroscopy; GFP, green fluorescent protein; GSD, ground state depletion; GSDIM, ground state depletion microscopy followed by individual molecule return; LTD, long-term depression; LTP, long-term potentiation; PALM, photoactivated localization microscopy; PSF, point-spread-function; RESOLFT, reversible saturable optical (fluorescence) transitions; rsgfp, reversible switchable GFP; SIM, structured-illumination microscopy; SMACM, single-molecule active control microscopy; SPT, singlemolecule or -particle tracking; STED, stimulated emission depletion; STORM, stochastic optical reconstruction microscopy; YFP, yellow fluorescent protein. 203
2 204 C. Eggeling et al. Photoactivated Localization Microscopy ((f)palm) (Betzig et al. 2006; Hess et al. 2006). Detailed reviews of the different nanoscopy approaches, especially of their different technical aspects, can be found elsewhere (Hell 2003, 2007, 2009a, b; Hell et al. 2004; Moerner 2006; Heintzmann and Ficz 2007; Rice 2007; Bates et al. 2008; Dedecker et al. 2008; Fernandez-Suarez and Ting 2008; Chi 2009; Evanko 2009; Heilemann et al. 2009; Heintzmann and Gustafsson 2009; Huang et al. 2009, 2010; Lippincott-Schwartz and Manley 2009; Huang 2010; Patterson et al. 2010; Dempsey et al. 2011; Muller et al. 2012). After a brief overview of these approaches, we will restrict ourselves to the basic principles underlying recent developments in optical nanoscopy which make it a highly versatile tool for the observation of the living cell, taking STED nanoscopy as an example and focusing on neurobiology and plasma membrane dynamics. The principle of fluorescence nanoscopy Differing in their technical details, all of the aforementioned fluorescence nanoscopy approaches are based on the principle of reversibly inhibiting the fluorescence emission of the cellular labels (Hell et al. 2003; Hell 2004, 2007) (Fig. 1). STED (Hell and Wichmann 1994; Klar et al. 2000), GSD (Hell and Kroug 1995; Bretschneider et al. 2007; Han et al. 2010) or more generally RESOLFT (Hell et al. 2003; Hofmann et al. 2005; Grotjohann et al. 2011) nanoscopes use a targeted or deterministic approach by inhibiting the fluorescence at pre-defined spatial positions, as outlined in the examples in Fig. 1a: a laser beam that excites fluorescence, that is, which switches fluorescence ON, is focused onto a small (diffraction-limited) spot. Scanning this spot over the sample and detecting the fluorescence for each scanning position permits the reconstruction of an image by providing the spatial distribution of the fluorescent-labeled molecules (as is for example realized in a confocal microscope). The spatial resolution is given by the so-called pointspread-function (PSF) of the microscope, which for the scanning microscope is mainly determined by the size of the laser focus. Unfortunately, the diameter of the spot onto which the light can be focused is determined by the diffraction of the light at the limited aperture of the microscope s (objective) lens approximately 200 nm for visible light (Abbe 1873; Hell 2007). However, addition of a second laser beam that switches off the fluorescence emission permits the observation of spots of nanoscopic size. Featuring a focal intensity distribution with at least one local intensity minimum (a zero-intensity point, e.g., at the focal center) this OFF light beam selectively inhibits fluorescence everywhere but at the zero-intensity point. Scanning fluorescent images therefore constitute a huge improvement in spatial resolution. Reversible inhibition of fluorescence is realized by, for example, stimulated emission (STED), photoswitchable fluorophores (RESOLFT) or transient transitions to the fluorescent labels metastable dark states (GSD). In 2006, a new approach arose, referred to under a multitude of different acronyms such as STORM (Rust et al. 2006), (f)palm (Betzig et al. 2006; Hess et al. 2006), Ground State Depletion Microscopy followed by individual molecule return (GSDIM) (F olling et al. 2008), direct STORM (dstorm) (Heilemann et al. 2008), or Single- Molecule Active Control Microscopy (SMACM) (Biteen et al. 2008). This approach relies on the stochastic on- and off-switching of the fluorescence emission of individual, well-separated labels combined with the determination of their exact spatial positions (Fig. 1b): usually, a large area of the sample is illuminated all at once and the spatial distribution of labeled molecules is determined by imaging the fluorescence onto a camera. Again, alike objects closer together than 200 nm cannot be distinguished, since because of the diffraction of light the image of a single point (PSF) appears blurred on the camera. By inhibiting the fluorescence emission of most of the labels, only single isolated molecules are allowed to fluoresce at a given time, and their spatial positions can be precisely determined from their blurred image spots on the camera using different localization approaches. By stochastically switching on and off different single isolated molecules in subsequent camera recordings, the final image with sub-diffraction sized spatial resolution is reconstructed from the summation of all localized spatial positions. It should be kept in mind that localization per se cannot provide sub-diffraction spatial resolution: separating alike objects at small distances requires a criterion to discern them from one another, such as switching them on and off in space and/or time (Hell and Wichmann 1994; Hell and Kroug 1995; Gordon et al. 2004; Qu et al. 2004; Lidke et al. 2005; Hell 2009b). This is why although it had been routinely applied for decades (see, e.g., Kusumi et al. 2005), specifically for spatio-temporal tracking of single isolated molecules, localization microscopy on its own did not provide images with sub-diffraction resolution. The different nanoscopy techniques can record images with both multiple colors (e.g., Donnert et al. 2007; Bock et al. 2007; Bates et al. 2007; Schmidt et al. 2008; Huang et al. 2008; Juette et al. 2008; Shtengel et al. 2009; Pavani et al. 2009) and improved spatial resolution along all spatial directions (e.g., Klar et al. 2000; Harke et al. 2008; Schmidt et al. 2008). However, they each have their advantages and disadvantages with respect to for example photobleaching, set-up complexity, image acquisition speed and image reconstruction. For instance, a direct image of the spatial distribution of labeled molecules is formed in the scanning approach of the deterministic STED, GSD and RESOLFT nanoscopes, whereas a reconstruction algorithm based on the identification and localization of single isolated molecules needs to be employed to create the final image in the stochastic (d)storm/(f)palm/gsdim
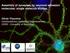
3 Superresolution in neurobiology and membrane dynamics 205 Fig. 1 Principle of far-field fluorescence nanoscopy. As a result of the diffraction of light, alike objects closer together than about 200 nm cannot be discerned when imaged with focused light [point-spreadfunction (PSF) ~ 200 nm in diameter], resulting in blurred images (left). Sub-diffraction images are realized by switching fluorescence on and off either in a deterministic or stochastic manner (middle), resulting in the ability to resolve structures < 200 nm in size (right). In the usual application of deterministic methods such as STED, GSD or RESOL- FT, a diffraction-limited spot of the fluorescence excitation (ON) laser beam is overlaid with a second laser beam which switches off fluorescence emission and which features at least one zero-intensity point. Consequently, the off light selectively inhibits fluorescence everywhere but at the zero-intensity points, creating observation spots of nanoscopic size. Scanning fluorescence images therefore feature a considerable improvement in spatial resolution. Stochastic methods such as (f)palm, (d)storm, GSDIM are usually based on the recording of multiple wide-field images using a sensitive camera. During these recordings only single isolated molecules are allowed to fluoresce, and their position is determined using different localization approaches. By stochastically switching on and off all molecules during the recordings, the final image with sub-diffraction sized spatial resolution is reconstructed from the summation of all localized spatial positions. approaches. Potential bias to the latter reconstruction process may arise for example from a lack of knowledge of missed or repeated recordings of molecules (Shroff et al. 2008; Small 2009). Furthermore, the determination of molecular positions may be biased for out-of-focus molecules (Enderlein et al. 2006; Engelhardt et al. 2011). Quite a number of improvements in the identification and localization algorithms have therefore been developed over the past years, especially aimed at image reconstructions for more densely labeled samples and/or those having low signal-to-noise levels (see, Dertinger et al. 2009; Cronin et al. 2009; Hedde et al. 2009; Smith et al. 2010; Larson 2010; Laurence and Chromy 2010; Henriques et al. 2010; Mortensen et al. 2010; Pertsinidis et al. 2010; Wolter et al. 2010; Endesfelder et al. 2010; Holden et al. 2011; Huang et al. 2011; Jones et al. 2011; Cox et al. 2012). Photobleaching of the fluorescent labels is a more complex issue in deterministic-based nanoscopy: whereas in stochastic-based methods a molecule needs in principle to be switched on and off only once, the fluorescence has to be switched on and off multiple times during the scanning recording of the deterministic nanoscopy approaches, favoring labels and experimental conditions that supply this condition we will come back to this issue later on. On the other hand, the image acquisition time of the stochastic (d) STORM/(f)PALM/GSDIM approaches is limited by the need to acquire sufficient molecular positions for the image reconstruction. Despite the aforementioned recent improvements in reconstruction algorithms there will potentially always be uncertainty as to whether the position of all molecules has been recorded, that is, of when to stop the acquisition, simply because these approaches are based on the stochastic on/off switching of fluorescence (see, e.g., Shroff et al. 2008; Small 2009). This is different for deterministic-based STED/GSD/RESOLFT approaches, where the scanning process defines the acquisition time. The use of fast beam scanners has established STED/ RESOLFT nanoscopy as the currently fastest sub-diffraction imaging technique available, with up to frames per second recording times for a few lm-sized observation areas (Westphal et al. 2008; Testa et al. 2012). In particular, the parallelization of the scanning procedure, that is, the
4 206 C. Eggeling et al. simultaneous scanning of multiple observation spots (such as realized for a structured illumination), leaves room for further improvement in the image acquisition speed of deterministic approaches (Heintzmann et al. 2002; Hell et al. 2003; Gustafsson 2005; Schwentker et al. 2007; Rego et al. 2012). The basic nanoscopy setups are usually expansions of conventional wide-field or (confocal) scanning microscopes by addition of an extra laser for switching fluorescence [note that confocality is not a requirement for the STED/GSD/RESOLFT concepts (Hell 2007)]. An initial concern of STED nanoscopy was the requirement for large, complex laser systems combined with the elaborate experimental procedure required to create zero-intensity point(s). However, recent advances in optical design and laser technology have permitted considerable simplification of the setups (Willig et al. 2007; Wildanger et al. 2008, 2009; Schrof et al. 2011; Vicidomini et al. 2011; Gould et al. 2012; Honigmann et al. 2012a; Mueller et al. 2012). Most importantly, as they are based on the same principle of switching fluorescence on and off, the very same sample can be used for both targeted- and stochastic-based nanoscopy (Bretschneider 2007; F olling et al. 2008; Brakemann et al. 2011). In view of the increased sensitivity and the aforementioned potential bias, both approaches can consequently be applied for the validation of results. Other approaches such as structured-illumination microscopy (SIM) (Gustafsson 2000; Schermelleh et al. 2008) or 4-Pi microscopy (Hell 1992; Gustafsson et al. 1995) are also often referred to as super-resolution microscopies. However, their spatial resolution is still limited (twofold improvement in spatial resolution for SIM and five to sixfold improvement in axial resolution for 4-Pi), that is they push the spatial resolution to the limits of the diffraction barrier. However, only the combination with reversible fluorescence inhibition processes can break these limits (Dyba and Hell 2002; Heintzmann et al. 2002; Hell et al. 2003; Gustafsson 2005; Schwentker et al. 2007; Rego et al. 2012). The fact that all the microscopy approaches are complementary, whether they are diffraction-limited or with nanoscale resolution, promotes research environments with access to various kinds of microscopes and nanoscopes, depending on their suitability for the case in hand. STED nanoscopy: the road to live cell imaging STED nanoscopy was not only the first nanoscopy technique to be developed, it was also the first to achieve cellular images with sub-diffraction spatial resolution (see, Klar et al. 2000; Willig et al. 2006b). Although a multitude of experiments over the past years have rendered STED imaging of fixed cells with down to 20 nm spatial resolution a generic tool for cellular studies, the potential phototoxic effect of the added STED light was long believed to be non-compatible with the study of living cells. Whereas the light intensities of the excitation laser are the same as in conventional confocal microscopy (1 10 lw), stimulated emission for example requires quite high light intensities of 1 10 MW/cm 2 at around nm of the added STED laser (for example supplied by mw average power of ~ pslong pulses of a titanium:sapphire laser), introducing the need to pay heed to heat absorption and light-induced toxic reactions. However, the involved peak intensities are smaller than those used in two-photon microscopy (Denk et al. 1990), a well-established tool in live-cell microscopy (Denk 1996). Further concerns about the addition of a STED laser arise from the increased photobleaching of the fluorescent label. This derives from the fact that the STED light acts on the excited fluorescent label. STED light per se is however not absorbed by the label and does not produce any photoreactive and thus -toxic species (unlike two-photon microscopy). Rather, stimulated emission shortens the time the fluorescent label spends in its excited (reactive) state and can reduce photoreactions such as photobleaching [compare with (Eggeling et al. 2005)]. Although excited state absorption of STED light can unfortunately cause severe photobleaching (Eggeling et al. 1998; Hotta et al. 2010), it was shown that fast scanning and the right choice of STED wavelength can minimize these effects (Donnert et al. 2009; Hotta et al. 2010; Moneron et al. 2010; Rankin et al. 2011). This is especially the case when using STED light in the range of 600 nm as required for live-cell labels such as green fluorescent protein (GFP) and its variants (Moneron et al. 2010; Rankin et al. 2011). Further, the use of a continuouswave laser in conjunction with a gated fluorescence detection scheme has enabled a reduction in the STED laser power and a simplification of the setups (Vicidomini et al. 2011; Honigmann et al. 2012a; Mueller et al. 2012). As a consequence, STED microscopy is nowadays considered a straightforward technique for the study of the living cell using genetically encoded markers such as fluorescent proteins (Willig et al. 2006a; Hein et al. 2008; Nagerl et al. 2008; Eggeling et al. 2009; Li et al. 2009; Moneron and Hell 2009; Morozova et al. 2010; Rankin et al. 2011; Tonnesen et al. 2011; Urban et al. 2011), tagging proteins such as SNAP-, HALO-, or CLIP-tags (Schr oder et al. 2008; Eggeling et al. 2009; Hein et al. 2010; Pellett et al. 2011; Lukinavicius et al. 2012), or fluorogen-activating tags (Fitzpatrick et al. 2009) (which both covalently bind functionalized and membrane-permeable organic dyes), even using commercial instrumentation (Schr oder et al. 2008; Fitzpatrick et al. 2009; Morozova et al. 2010; Friedemann et al. 2011). Furthermore, the quite high laser intensities of 1 10 MW/cm 2 can be circumvented when switching from STED to RESOLFT nanoscopy, which employs laser intensities in the range of 1 5 kw/cm 2 only (Hofmann et al. 2005; Brakemann et al. 2011; Grotjohann et al. 2011; Testa et al. 2012). However, in contrast to STED, which can in principle employ any label, RESOLFT nanoscopy relies on
5 Superresolution in neurobiology and membrane dynamics 207 the use of special reversible photoswitchable molecules such as the reversible switchable GFP (rsgfp) (Grotjohann et al. 2011). Fortunately, the number of reversible switchable fluorescent proteins has significantly increased over the last years and their switching characteristics steadily improved (Grotjohann et al. 2012). Live-cell STED microscopy in neuroscience Confocal and two-photon microscopies have become common tools in Neurobiology, and have found wide application in the study of neuronal connections or processes, particularly those lying a few microns deep in, for example, cerebral cortex. It is important to study the neurons in living brain tissue and even the living animal, where all connections are preserved and functional. However, the diffraction-limited resolution of conventional far-field light microscopy is inadequate to resolve fine details, obliging researchers to complement the live-cell imaging with electron microscopy. This comes at a high cost, because the latter form of microscopy requires fixation, dehydration, embedding, thin cutting, and vacuum observation of the dehydrated nervous tissue. A case in point is the study of dendritic spines, the fine processes that form the post-synaptic compartment of central nervous system synapses. The majority of the excitatory synapses in the central nervous system are formed on dendritic spines. These micrometer/sub-micrometer-sized protrusions of the dendrite come in a variety of shapes: filopodia-like, stubby, cup-shaped, or mushroom shaped. Interestingly, dendritic spines show developmentally regulated and activity-dependent morphological changes. By adapting a STED microscope to the demands of in vivo imaging, the subtle dynamics of spines in the cerebral cortex of a living mouse were made visible (Fig. 2) (Berning et al. 2012). In living organotypic cultures of brain slices, it was shown that morphological parameters such as the neck width and curvature of the spine heads can be quantified to record changes, for example, induced by chemical long-term potentiation (cltp) (Nagerl et al. 2008). The imaging of sub-cellular structures is also made possible with STED microscopy: actin, a key player in the morphological changes of synapses, can be visualized by STED microscopy. Using a fusion protein of Lifeact (a small peptide label for actin that does not disturb the actin polymerization or dynamics) and the yellow fluorescent protein (YFP), dynamic changes in actin molecules in synaptic spines located up to 120 lm deep in the cerebral tissue were observed (Urban et al. 2011). Establishment of either LTP or long-term depression (LTD) apparently requires a responsive F-actin cytoskeleton (Yuste and Bonhoeffer 2001). During neuronal development, a healthy cytoskeleton is essential for brain neurons to undergo the sequential steps of neurogenesis, cell migration and terminal differentiation (Ik-Tsen Heng et al. 2009). Nanoscopy studies of the actin cytoskeleton is gaining momentum in work on dendritic spines in brain (Tatavarty et al. 2011; Berning et al. 2012), (Izeddin et al. 2011) and other cells and tissues (Xu et al. 2012). The localization of Na +,K + -ATPase has recently been accomplished using STED microscopy (Blom et al. 2011). Insight into the organization of protein complexes in the presynaptic active zone and post-synaptic density has also been gained with the aid of other nanoscopy techniques such as STORM (Dani et al. 2010). Endosomal Fig. 2 Live-cell STED nanoscopy. STED imaging of the temporal dynamics of dendritic processes within the molecular layer of a TgN (Thy1-YFP) mouse, about lm below the surface. Optical access to the visual cortex in the anesthetized mouse was provided by a glass sealed window. Scale bar: 1 lm. Adapted from (Berning et al. 2012).
6 208 C. Eggeling et al. sorting of vesicles in the presynapse has been analyzed in one of the few studies combining STED and electron microscopy (Hoopmann et al. 2010). For imaging deep inside living tissue, STED microscopy has natural advantages over stochastic-based nanoscopy such as (d)storm/(f)palm/gsdim. It provides sectioning capability and superior timing resolution. And, as shown by the various examples presented here, it can be a valuable tool in understanding basic working principles of the brain. Furthermore, it can be combined with two-photon excitation (Ding et al. 2009; Li et al. 2009; Moneron and Hell 2009; Bianchini and Diaspro 2012) or adaptive optics (Gould et al. 2012) for an optimized fluorescence excitation in deep tissue. Live-cell STED microscopy of plasma membrane organization A prominent example of the need for sub-diffraction resolution is the observation of plasma membrane heterogeneity such as in lipid protein interactions, which are considered to play a functional part in a wide range of membrane-associated functionally relevant processes (see, e.g., Lingwood and Simons 2010). Unfortunately, many structural characteristics of the cellular membrane such as protein clusters, areas of different molecular order (often denoted lipid rafts ), or clathrin-coated pits are < 200 nm in size. Thus, their direct and non-invasive observation in the living cell is impeded by the resolution limit of > 200 nm of the conventional far-field fluorescence microscope. Over the last years, we have published several reports on the detection of nanoscopic membrane heterogeneities in the plasma membrane of living cells using the superior spatial resolution of STED nanoscopy. Besides directly imaging the organization of < 70 nm large protein clusters in the plasma membrane (Sieber et al. 2007), we could obtain new details of molecular membrane dynamics by combining a (tunable) resolution of down to 30 nm with tools such as fluorescence correlation spectroscopy (FCS) (Eggeling et al. 2009; Ringemann et al. 2009; Mueller et al. 2011; Honigmann et al. 2012b; Sezgin et al. 2012) (Fig. 3). In FCS, the transits Fig. 3 Live-cell STED nanoscopy: STED-FCS analysis of lipid plasma membrane diffusion. (Upper panel) Lipids and proteins are heterogeneously distributed in the cellular plasma membrane, such distribution often stemming for example from cholesterol-assisted lipid protein interactions (which may be the basis for the coalescence of transient signaling platforms nanoscopic membrane domains termed lipid rafts, that is, spatially confined molecular assemblies of different lipids and proteins which are essential for certain transmembrane signaling events), or the underlying cytoskeleton (which is membrane-anchored via proteins). Adapted from (Lingwood and Simons 2010). (Lower left panel) The dependency of the transit time τ D for different subdiffraction sized observation areas ~ d 2 (as tuned by the STED laser power) shows an almost free diffusion (linear dependence, dark gray line diffusion coefficient 0.5 lm 2 /s) for a fluorescent phospholipid analogue (phosphoethanolamine, PE, red squares), and a hindered diffusion (non-linear dependence) for a fluorescent sphingolipid analogue (sphingomyelin, SM, gray circles). Depletion of cholesterol by cholesterol oxidase (COase) reduces hindrances in molecular diffusion of SM (SM+COase, open circles). The minimal change in τ D for very small observation areas (gray horizontal line) and Monte-Carlo simulations indicate that the hindrance in diffusion is caused by transient complexes with either relatively slow-moving or immobilized membrane molecules (red dotted line) and not by incorporation into 20 nm large domains, where diffusion is slowed down (green dotted line). Direct observation of these transient interactions is impossible with the large diffraction-limited confocal observation area (gray shaded area). (Lower right panel) Schematic drawing of normal free (red) and hindered SM diffusion (blue, dots: points of interactions or complexes).
7 Superresolution in neurobiology and membrane dynamics 209 of single molecules through a usually fixed observation spot are observed to determine dynamic parameters such as the average transit time (Magde et al. 1972; Ehrenberg and Rigler 1974; Haustein and Schwille 2003). Investigating the dependence of the average transit time on the size of the observation spot highlights hindrances in molecular diffusion such as transient molecular interactions (Wawrezinieck et al. 2005). STED nanoscopy is a perfect tool for these studies as it allows a straightforward variation of the observation spot size by, for example, variation of the power of the STED laser (Eggeling 2012). Our STED-FCS experiments revealed that sphingolipids and certain proteins are transiently (~ 10 ms) trapped on the nanoscale in often cholesterolmediated and/or cytoskeleton-associated molecular complexes, whereas other molecules diffuse freely (Mueller et al. 2011). Different lipid structures show up distinctly, but so far at least such differences do not appear to be because of variations in the labeling. We observed strong differences in the molecular nature behind the nanoscopic molecular interactions as observed by STED-FCS and the separation of membranes into liquid-disordered and -ordered phases (Mueller et al. 2011; Sezgin et al. 2012). Specifically the latter phase is often referred to as being an appropriate physical model of lipid nanodomains, the so-called lipid rafts. Together with a suitable fluorescent lipid marker for the liquid-ordered phase, STED-FCS has the potential to shed new light on the mystery and functionality of these lipid nanodomains (Honigmann et al. 2012b). STED-FCS is very similar to single-molecule or -particle tracking (SPT), which is also often applied to investigate diffusion characteristics of membrane components (e.g., Kusumi et al. 2005; Wieser et al. 2007; Manley et al. 2008; Sahl et al. 2010). While SPT allows the direct visualization of the diffusion path of individual molecules and thus of any (even less abundant) hindrances, it requires much longer recording times and more detailed data analysis than FCS to obtain average molecular characteristics of high statistical accuracy. Nevertheless, both STED-FCS and SPT, and more so their complementary applications, have the potential to revolutionize our understanding of membrane bioactivity (e.g., Kusumi et al. 2010; Eggeling 2012). Conclusions It is highly likely that the novel possibilities of far-field fluorescence STED nanoscopy described in this short review will contribute to our understanding of the living cell in general, and will shed new light on a whole range of cellular phenomena, including key neurobiological processes such as development, pruning, and stabilization of synapses, their subtle dynamic changes triggered by chemical or electrical stimulation, and the possible alteration of these processes in disease. Likewise, the new nanoscopies are ideally suited to address important issues in membrane biology which are bound to find answers at the nanoscale, such as lipid protein interactions and nanodomains in membrane bioactivity and receptor signaling in neurobiology and cell biology at large. Conflicts of interest The authors declare no conflicts of interest. References Abbe E. (1873) Beitr age zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Archiv f ur Mikroskopische Anatomie 9, Bates M., Huang B., Dempsey G. T. and Zhuang X. W. (2007) Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science 317, Bates M., Huang B. and Zhuang X. W. (2008) Super-resolution microscopy by nanoscale localization of photo-switchable fluorescent probes. Curr. Opin. Chem. Biol. 12, Berning S., Willig K. I., Steffens H., Dibaj P. and Hell S. W. (2012) Nanoscopy in a living mouse brain. Science 335, 551. Betzig E., Patterson G. H., Sougrat R., Lindwasser O. W., Olenych S., Bonifacino J. S., Davidson M. W., Lippincott-Schwartz J. and Hess H. F. (2006) Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, Bianchini P. and Diaspro A. (2012) Fast scanning STED and two-photon fluorescence excitation microscopy with continuous wave beam. J. Microsc. 245, Biteen J. S., Thompson M. A., Tselentis N. K., Bowman G. R., Shapiro L. and Moerner W. E. (2008) Super-resolution imaging in live caulobacter crescentus cells using photoswitchable EYFP. Nat. Methods 5, Blom H., R onnlund D., Scott L., Spicarova Z., Widengren J., Bondar A., Aperia A. and Brismar H. (2011) Spatial distribution of Na+-K + - ATPase in dendritic spines dissected by nanoscale superresolution STED microscopy. BMC Neurosci. 12, 16. Bock H., Geisler C., Wurm C. A., Von Middendorff C., Jakobs S., Schonle A., Egner A., Hell S. W. and Eggeling C. (2007) Twocolor far-field fluorescence nanoscopy based on photoswitchable emitters. Appl. Phys. B 88, Brakemann T., Stiel A. C., Weber G. et al. (2011) A reversibly photoswitchable GFP-like protein with fluorescence excitation decoupled from switching. Nat. Biotechnol. 29, Bretschneider S. (2007) Ground-State-Depletion Fluorescence Microscopy. University Goettingen, Goettingen. Bretschneider S., Eggeling C. and Hell S. W. (2007) Breaking the diffraction barrier in fluorescence microscopy by optical shelving. Phys. Rev. Lett. 98, Chi K. R. (2009) Super-resolution microscopy: breaking the limits. Nat. Methods 6, Cox S., Rosten E., Monypenny J., Jovanovic-Talisman T., Burnette D. T., Lippincott-Schwartz J., Jones G. E. and Heintzmann R. (2012) Bayesian localization microscopy reveals nanoscale podosome dynamics. Nat. Methods 9, Cronin B., de Wet B. and Wallace M. I. (2009) Lucky imaging: improved localization accuracy for single molecule imaging. Biophys. J. 96, Dani S., Huang B., Bergan J., Dulac C. and Zhuang X. (2010) Superresolution imaging of chemical synapses in the brain. Neuron 68, Dedecker P., Hofkens J. and Hotta J. I. (2008) Diffraction-unlimited optical microscopy. Mater. Today 11,
8 210 C. Eggeling et al. Dempsey G. T., Vaughan J. C., Chen K. H., Bates M. and Zhuang X. (2011) Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat. Methods 8, Denk W. (1996) Two-photon excitation in functional biological imaging. J. Biomed. Opt. 1, Denk W., Strickler J. H. and Webb W. W. (1990) 2-photon laser scanning fluorescence microscopy. Science 248, Dertinger T., Colyer R., Iyer G., Weiss S. and Enderlein J. (2009) Fast, background-free, 3D super-resolution optical fluctuation imaging (SOFI). PNAS 106, Ding J. B., Takasaki K. T. and Sabatini B. L. (2009) Supraresolution imaging in brain slices using stimulated-emission depletion twophoton laser scanning microscopy. Neuron 63, Donnert G., Keller J., Wurm C. A. et al. (2007) Two-color far-field fluorescence nanoscopy. Biophys. J. 92, L67 L69. Donnert G., Eggeling C. and Hell S. W. (2009) Triplet-relaxation microscopy with bunched pulsed excitation. Photochem. Photobiol. 8, Dyba M. and Hell S. W. (2002) Focal spots of size lambda/23 open up far-field florescence microscopy at 33 nm axial resolution. Phys. Rev. Lett. 88, Eggeling C. (2012) STED-FCS nanoscopy of membrane dynamics, in Fluorescent Methods to Study Biological Membranes (Mely Y. and Duportail G., eds), pp Springer-Verlag, Berlin. Eggeling C., Widengren J., Rigler R. and Seidel C. A. M. (1998) Photobleaching of fluorescent dyes under conditions used for single-molecule detection: Evidence of two-step photolysis. Anal. Chem. 70, Eggeling C., Volkmer A. and Seidel C. A. M. (2005) Molecular photobleaching kinetics of Rhodamine 6G by one- and two-photon induced confocal fluorescence microscopy. ChemPhysChem 6, Eggeling C., Ringemann C., Medda R. et al. (2009) Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457, 1159 U1121. Ehrenberg M. and Rigler R. (1974) Rotational brownian motion and fluorescence intensity fluctuations. Chem. Phys. 4, Enderlein J., Toprak E. and Selvin P. R. (2006) Polarization effect on position accuracy of fluorophore localization. Opt. Express 14, Endesfelder U., van de Linde S., Wolter S., Sauer M. and Heilemann M. (2010) Subdiffraction-resolution fluorescence microscopy of myosin actin motility. ChemPhysChem 11, Engelhardt J., Keller J., Hoyer P., Reuss M., Staudt T. and Hell S. W. (2011) Molecular orientation affects localization accuracy in superresolution far-field fluorescence microscopy. Nano Lett. 11, Evanko D. (2009) Primer: fluorescence imaging under the diffraction limit. Nat. Methods 6, Fernandez-Suarez M. and Ting A. Y. (2008) Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 9, Fitzpatrick J. A., Yan Q., Sieber J. J. et al. (2009) STED nanoscopy in living cells using fluorogen activating proteins. Bioconjug. Chem. 20, F olling J., Bossi M., Bock H., Medda R., Wurm C. A., Hein B., Jakobs S., Eggeling C. and Hell S. W. (2008) Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat. Methods 5, Friedemann K., Turshatov A., Landfester K. and Crespy D. (2011) Characterization via two-color STED microscopy of nanostructured materials synthesized by colloid electrospinning. Langmuir 27, Gordon M. P., Ha T. and Selvin P. R. (2004) Single-molecule highresolution imaging with photobleaching. PNAS 101, Gould T. J., Burke D., Bewersdorf J. and Booth M. J. (2012) Adaptive optics enables 3D STED microscopy in aberrating specimens. Opt. Express 20, Grotjohann T., Testa I., Leutenegger M. et al. (2011) Diffractionunlimited all-optical imaging and writing with a photochromic GFP. Nature 478, Grotjohann T., Testa I., Reuss M., Brakemann T., Eggeling C., Hell S. W. and Jakobs S. (2012) rsegfp2 enables fast RESOLFT nanoscopy of living cells. Elife 1, e Gustafsson M. G. L. (2000) Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, Gustafsson M. G. L. (2005) Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimited resolution. PNAS 102, Gustafsson M. G. L., Agard D. A. and Sedat J. W. (1995) Sevenfold improvement of axial resolution in 3D widefield microscopy using two objective lenses. Proc. Soc. Photo Opt. Instrum. Eng. 2412, Han K. Y., Kim S. K., Eggeling C. and Hell S. (2010) Metastable dark states enable ground state depletion microscopy of nitrogen vacancy centers in diamond with diffraction-unlimited resolution. Nano Lett. 10, Harke B., Ullal C. K., Keller J. and Hell S. W. (2008) Three-dimensional nanoscopy of colloidal crystals. Nano Lett. 8, Haustein E. and Schwille P. (2003) Ultrasensitive investigations of biological systems by fluorescence correlation spectroscopy. Methods 29, Hedde P. N., Fuchs J., Oswald F., Wiedenmann J. and Nienhaus G. U. (2009) Online image analysis software for photoactivation localization microscopy. Nat. Methods 6, Heilemann M., van de Linde S., Schuttpelz M., Kasper R., Seefeldt B., Mukherjee A., Tinnefeld P. and Sauer M. (2008) Subdiffractionresolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. Engl. 47, Heilemann M., Dedecker P., Hofkens J. and Sauer M. (2009) Photoswitches: key molecules for subdiffraction-resolution fluorescence imaging and molecular quantification. Laser Photon. Rev. 3, Hein B., Willig K. I. and Hell S. W. (2008) Stimulated emission depletion (sted) nanoscopy of a fluorescent protein-labeled organelle inside a living cell. PNAS 105, Hein B., Willig K. I., Wurm C. A., Westphal V., Jakobs S. and Hell S. W. (2010) Stimulated emission depletion nanoscopy of living cells using snap-tag fusion proteins. Biophys. J. 98, Heintzmann R. and Ficz G. (2007) Breaking the resolution limit in light microscopy. Methods Cell Biol. 81, Heintzmann R. and Gustafsson M. G. L. (2009) Subdiffraction resolution in continuous samples. Nat. Photonics 3, Heintzmann R., Jovin T. M. and Cremer C. (2002) Saturated patterned excitation microscopy - A concept for optical resolution improvement. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 19, Hell S. W. (1992) Double-scanning confocal microscope. European Patent, Vol Hell S. W. (2003) Toward fluorescence nanoscopy. Nat. Biotechnol. 21, Hell S. W. (2004) Strategy for far-field optical imaging and writing without diffraction limit. Phys. Lett. A 326, Hell S. W. (2007) Far-field optical nanoscopy. Science 316, Hell S. (2009a) Far-field optical nanoscopy, in Single Molecule Spectroscopy in Chemistry (Gr aslund A., Rigler R. and Widengren J., eds), pp Springer, Berlin.
9 Superresolution in neurobiology and membrane dynamics 211 Hell S. W. (2009b) Microscopy and its focal switch. Nat. Methods 6, Hell S. W. and Kroug M. (1995) Ground-state depletion fluorescence microscopy, a concept for breaking the diffraction resolution limit. Appl. Phys. B 60, Hell S. W. and Wichmann J. (1994) Breaking the diffraction resolution limit by stimulated-emission - stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, Hell S. W., Jakobs S. and Kastrup L. (2003) Imaging and writing at the nanoscale with focused visible light through saturable optical transitions. Appl. Phys. A 77, Hell S. W., Dyba M. and Jakobs S. (2004) Concepts for nanoscale resolution in fluorescence microscopy. Curr. Opin. Neurobiol. 14, Henriques R., Lelek M., Fornasiero E. F., Valtorta F., Zimmer C. and Mhlanga M. M. (2010) QuickPALM: 3D real-time photoactivation nanoscopy image processing in ImageJ. Nat. Methods 7, Hess S. T., Girirajan T. P. K. and Mason M. D. (2006) Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 91, Hofmann M., Eggeling C., Jakobs S. and Hell S. W. (2005) Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. PNAS 102, Holden S. J., Uphoff S. and Kapanidis A. N. (2011) DAOSTORM: an algorithm for highdensity super-resolution microscopy. Nat. Methods 8, Honigmann A., Eggeling C., Schulze M. and Lepert A. (2012a) Superresolution STED microscopy advances with yellow CW OPSL. Laser Focus World 48, Honigmann A., Mueller V., Hell S. W. and Eggeling C. (2012b) STED microscopy detects and quantifies liquid phase separation in lipid membranes using a new far-red emitting fluorescent phosphoglycerolipid analogue. Faraday Discussions 161, doi: /c2fd20107k. Hoopmann P., Punge A., Baryscha S. V. et al. (2010) Endosomal sorting of readily releasable synaptic vesicles. PNAS 107, Hotta J., Fron E., Dedecker P. et al. (2010) Spectroscopic Rationale for efficient stimulated-emission depletion microscopy fluorophores. J. Am. Chem. Soc. 132, Huang B. (2010) Super-resolution optical microscopy: multiple choices. Curr. Opin. Chem. Biol. 14, Huang B., Wang W. Q., Bates M. and Zhuang X. W. (2008) Threedimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, Huang B., Bates M. and Zhuang X. (2009) Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 78, Huang B., Babcock H. and Zhuang X. (2010) Breaking the diffraction barrier: super-resolution imaging of cells. Cell 143, Huang F., Schwartz S. L., Byars J. M. and Lidke K. A. (2011) Simultaneous multiple-emitter fitting for single molecule superresolution imaging. Biomed. Opt. Express 2, Ik-Tsen Heng J., Chariot A. and Nguyen L. (2009) Molecular layers underlying cytoskeletal remodelling during cortical development. Trends Neurosci. 33, Izeddin I., Specht C. G., Lelek M., Darzacq X., Triller A., Zimmer C. and Dahan M. (2011) Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS ONE 6, e Jones S. A., Shim S.-H., He J. and Zhuang X. (2011) Fast, threedimensional super-resolution imaging of live cells. Nat. Methods 8, Juette M. F., Gould T. J., Lessard M. D., Mlodzianoski M. J., Nagpure B. S., Bennett B. T., Hess S. T. and Bewersdorf J. (2008) Threedimensional sub-100 nm resolution fluorescence microscopy of thick samples. Nat. Methods 5, Klar T. A., Jakobs S., Dyba M., Egner A. and Hell S. W. (2000) Fluorescence microscopy with diffraction resolution barrier broken by stimulated emission. PNAS 97, Kusumi A., Nakada C., Ritchie K., Murase K., Suzuki K., Murakoshi H., Kasai R. S., Kondo J. and Fujiwara T. (2005) Paradigm shift of the plasmamembrane concept from the two-dimensional continuum fluid to the partitioned fluid: High-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. 34, Kusumi A., Shirai Y. M., Koyama-Honda I., Suzuki K. G. N. and Fujiwara T. K. (2010) Hierarchical organization of the plasma membrane: investigations by single-molecule tracking vs. fluorescence correlation spectroscopy. FEBS Lett. 584, Larson D. R. (2010) The economy of photons. Nat. Methods 7, Laurence T. A. and Chromy B. A. (2010) Efficient maximum likelihood estimator fitting of histograms. Nat. Methods 5, Li Q., Wu S. S. H. and Chou K. C. (2009) Subdiffraction-limit twophoton fluorescence microscopy for GFP-tagged cell imaging. Biophys. J. 97, Lidke K. A., Rieger B., Jovin T. M. and Heintzmann R. (2005) Superresolution by localization of quantum dots using blinking statistics. Opt. Express 13, Lingwood D. and Simons K. (2010) Lipid rafts as a membraneorganizing principle. Science 327, Lippincott-Schwartz J. and Manley S. (2009) Putting super-resolution fluorescence microscopy to work. Nat. Methods 6, Lukinavicius G., Umezawa K., Olivier N. et al. (2012) A near-infrared fluorophore for live-cell superresolution microscopy of cellular proteins. Nat. Chem. 5, doi: /nchem Magde D., Webb W. W. and Elson E. (1972) Thermodynamic fluctuations in a reacting system - measurement by fluorescence correlation spectroscopy. Phys. Rev. Lett. 29, Manley S., Gillette J. M., Patterson G. H., Shroff H., Hess H. F., Betzig E. and Lippincott-Schwartz J. (2008) High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, Moerner W. E. (2006) Single-molecule mountains yield nanoscale cell images. Nat. Methods 3, Moneron G. and Hell S. (2009) Two-photon excitation STED microscopy. Opt. Express 17, Moneron G., Medda R., Hein B., Giske A., Westphal V. and Hell S. W. (2010) Fast STED microscopy with continuous wave fiber lasers. Opt. Express 18, Morozova K. S., Piatkevich K. D., Gould T. J., Zhang J., Bewersdorf J. and Verkhusha V. V. (2010) Far-red fluorescent protein excitable with red lasers for flow cytometry and superresolution STED nanoscopy. Biophys. J. 99, L13 L15. Mortensen K. I., Churchman S. L., Spudich J. A. and Flyvbjerg H. (2010) Optimized localization analysis for single-molecule tracking and super-resolution microscopy. Nat. Methods 7, Mueller V., Ringemann C., Honigmann A. et al. (2011) STED nanoscopy reveals molecular details of cholesterol- and cytoskeleton-modulated lipid interactions in living cells. Biophys. J. 101, Mueller V., Eggeling C., Karlsson H. and von Gegerfelt D. (2012) CW DPSS lasers make STED microscopy more practical. Biophotonics 19, Muller T., Schumann C. and Kraegeloh A. (2012) STED Microscopy and its applications: new insights into cellular processes on the nanoscale. ChemPhysChem 13, Nagerl U. V., Willig K. I., Hein B., Hell S. W. and Bonhoeffer T. (2008) Live-cell imaging of dendritic spines by STED microscopy. PNAS 105,
10 212 C. Eggeling et al. Patterson G., Davidson M., Manley S. and Lippincott-Schwartz J. (2010) Superresolution imaging using single-molecule localization. Annu. Rev. Phys. Chem. 61, Pavani S. R. P., Thompson M. A., Biteen J. S., Lord S. J., Liu N., Twieg R. J., Piestun R. and Moerner W. E. (2009) Three-dimensional, single-molecule fluorescence imaging beyond the diffraction limit by using a double-helix point spread function. PNAS 106, Pellett P. A., Sun X., Gould T. J., Rothman J. E., Xu M.-Q., Correa I. R. Jr and Bewersdorf J. (2011) Two-color STED microscopy in living cells. Biomed. Opt. Express 2, Pertsinidis A., Zhang Y. and Chu S. (2010) Subnanometre singlemolecule localization, registration and distance measurements. Nature 466, Qu X. H., Wu D., Mets L. and Scherer N. F. (2004) Nanometer-localized multiple single-molecule fluorescence microscopy. PNAS 101, Rankin B. R., Moneron G., Wurm C. A., Nelson J. C., Walter A., Schwarzer D., Schroeder J., Colon-Ramos D. A. and Hell S. W. (2011) Nanoscopy in a living multicellular organism expressing GFP. Biophys. J. 100, L63 L65. Rego E. H., Shao L., Macklin J. J., Winoto L., Johansson G. A., Kamps- Hughes N., Davidson M. W. and Gustafsson M. G. L. (2012) Nonlinear structured-illumination microscopy with a photoswitchable protein reveals cellular structures at 50-nm resolution. PNAS 109, E135 E143. Rice J. H. (2007) Beyond the diffraction limit: far-field fluorescence imaging with ultrahigh resolution. Mol. BioSyst. 3, Ringemann C., Harke B., Middendorff C. V. et al. (2009) Exploring single-molecule dynamics with fluorescence nanoscopy. New J. Phys. 11, Rust M. J., Bates M. and Zhuang X. W. (2006) Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, Sahl S. J., Leutenegger M., Hilbert M., Hell S. W. and Eggeling C. (2010) Fast molecular tracking maps nanoscale dynamics of plasma membrane lipids. PNAS 107, Schermelleh L., Carlton P. M., Haase S. et al. (2008) Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320, Schmidt R., Wurm C. A., Jakobs S., Engelhardt J., Egner A. and Hell S. W. (2008) Spherical nanosized focal spot unravels the interior of cells. Nat. Methods 5, Schr oder J., Benink H., Dyba M. and Los G. V. (2008) In vivo labeling method using a genetic construct for nanoscale resolution microscopy. Biophys. J. 96, L1 L3. Schrof S., Staudt T., Rittweger E., Wittenmayer N., Dresbach T., Engelhardt J. and Hell S. W. (2011) STED nanoscopy with massproduced laser diodes. Opt. Express 19, Schwentker M. A., Bock H., Hofmann M., Jakobs S., Bewersdorf J., Eggeling C. and Hell S. W. (2007) Wide-field subdiffraction RESOLFT microscopy using fluorescent protein photoswitching. Microsc. Res. Tech. 70, Sezgin E., Levental I., Grzybek M. et al. (2012) Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes.. Biochimica et Biophysica Acta (BBA) - Biomembranes 1818, Shroff H., Galbraith C. G., Galbraith J. A. and Betzig E. (2008) Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat. Methods 5, Shtengel G., Galbraith J. A., Galbraith C. G. et al. (2009) Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. PNAS 106, Sieber J. J., Willig K. I., Kutzner C. et al. (2007) Anatomy and dynamics of a supramolecular membrane protein cluster. Science 317, Small A. R. (2009) Theoretical limits on errors and acquisition rates in localizing switchable fluorophores. Biophys. J. 96, L16 L18. Smith C. S., Joseph N., Rieger B. and Lidke K. A. (2010) Fast, singlemolecule localization that achieves theoretically minimum uncertainty. Nat. Methods 7, Tatavarty V., Eun-Ji Kim E.-J., Rodionov V. and Yu J. (2011) Investigating sub-spine actin dynamics in rat hippocampal neurons with super-resolution optical imaging. PLoS ONE 4, e7724. Testa I., Urban N. T., Jakobs S., Eggeling C., Willig K. I. and Hell S. W. (2012) Nanoscopy of living brain slices with low light levels. Neuron 75, Tonnesen J., Nadrigny F., Willig K. I., Wedlich-Soldner R. and Nagerl U. V. (2011) Two-color STED microscopy of living synapses using a single laser-beam pair. Biophys. J. 101, Urban N. T., Willig K. I., Hell S. W. and Nagerl U. V. (2011) STED Nanoscopy of actin dynamics in synapses deep inside living brain slices. Biophys. J. 101, Vicidomini G., Moneron G., Han K. Y., Westphal V., Ta H., Reuss M., Engelhardt H., Eggeling C. and Hell S. W. (2011) Sharper lowpower STED nanoscopy by time gating. Nat. Methods 8, Wawrezinieck L., Rigneault H., Marguet D. and Lenne P. F. (2005) Fluorescence correlation spectroscopy diffusion laws to probe the submicron cell membrane organization. Biophys. J. 89, Westphal V., Rizzoli S. O., Lauterbach M. A., Kamin D., Jahn R. and Hell S. W. (2008) Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science 320, Wieser S., Moertelmaier M., Fuertbauer E., Stockinger H. and Schutz G. (2007) (Un)Confined diffusion of CD59 in the plasma membrane determined by high-resolution single molecule microscopy. Biophys. J. 92, Wildanger D., Rittweger E., Kastrup L. and Hell S. W. (2008) STED microscopy with a supercontinuum laser source. Opt. Express 16, Wildanger D., B uckers J., Westphal V., Hell S. W. and Kastrup L. (2009) A STED microscope aligned by design. Opt. Express 17, Willig K. I., Kellner R. R., Medda R., Hein B., Jakobs S. and Hell S. W. (2006a) Nanoscale resolution in GFP-based microscopy. Nat. Methods 3, Willig K. I., Rizzoli S. O., Westphal V., Jahn R. and Hell S. W. (2006b) Sted microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature 440, Willig K. I., Harke B., Medda R. and Hell S. W. (2007) STED microscopy with continuous wave beams. Nat. Methods 4, Wolter S., Schuttpelz M., Tscherepanow M., van de Linde S., Heilemann M. and Sauer M. (2010) Real-time computation of subdiffractionresolution fluorescence images. J. Microsc. 237, Xu K., Babcock H. P. and Zhuang X. (2012) Dual-objective STORM reveals three-dimensional filament organization in the actin cytoskeleton. Nat. Methods 9, Yuste R. and Bonhoeffer T. (2001) Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 24,
STORM/PALM. Super Resolution Microscopy 10/31/2011. Looking into microscopic world of life
 Super Resolution Microscopy STORM/PALM Bo Huang Department of Pharmaceutical Chemistry, UCSF CSHL Quantitative Microscopy, 1/31/211 Looking into microscopic world of life 1 µm 1 µm 1 nm 1 nm 1 nm 1 Å Naked
Super Resolution Microscopy STORM/PALM Bo Huang Department of Pharmaceutical Chemistry, UCSF CSHL Quantitative Microscopy, 1/31/211 Looking into microscopic world of life 1 µm 1 µm 1 nm 1 nm 1 nm 1 Å Naked
Introduction CHAPTER 1
 CHAPTER 1 Introduction 1.1 Light Microscopy The light microscope is one of the significant inventions in the history of humankind that, along with the telescope, played a central role in the Scientific
CHAPTER 1 Introduction 1.1 Light Microscopy The light microscope is one of the significant inventions in the history of humankind that, along with the telescope, played a central role in the Scientific
SIM SSIM. nanoscopy RESOLFT. Super-resolution STORM GSDIM. dstorm PALMIRA FPALM PALM PAINT SPRAIPAINT SOFI BALM CALM. Bo Huang
 STEDGSD STORM SOFI nanoscopy GSDIM PALMIRA SMACM BBB PAINT SPRAIPAINT CALM RESOLFT BALM SIM SSIM Super-resolution Bo Huang 2013.08.01 dstorm FPALM PALM 50 years to extend the resolution Confocal microscopy
STEDGSD STORM SOFI nanoscopy GSDIM PALMIRA SMACM BBB PAINT SPRAIPAINT CALM RESOLFT BALM SIM SSIM Super-resolution Bo Huang 2013.08.01 dstorm FPALM PALM 50 years to extend the resolution Confocal microscopy
Super-resolution microscopy at a glance
 ARTICLE SERIES: Imaging Cell Science at a Glance 1607 Super-resolution microscopy at a glance Catherine G. Galbraith 1, * and James A. Galbraith 2, * 1 National Institute of Health, 1 NICHD and 2 NINDS,
ARTICLE SERIES: Imaging Cell Science at a Glance 1607 Super-resolution microscopy at a glance Catherine G. Galbraith 1, * and James A. Galbraith 2, * 1 National Institute of Health, 1 NICHD and 2 NINDS,
Fluorescence Nanoscopy
 Fluorescence Nanoscopy Keith A. Lidke University of New Mexico panda3.phys.unm.edu/~klidke/index.html Optical Microscopy http://en.wikipedia.org/wiki/k%c3%b6hler_illumination 30 µm Fluorescent Probes Michalet
Fluorescence Nanoscopy Keith A. Lidke University of New Mexico panda3.phys.unm.edu/~klidke/index.html Optical Microscopy http://en.wikipedia.org/wiki/k%c3%b6hler_illumination 30 µm Fluorescent Probes Michalet
New developments in STED Microscopy
 New developments in STED Microscopy Arnold Giske*, Jochen Sieber, Hilmar Gugel, Marcus Dyba, Volker Seyfried, Dietmar Gnass Leica Microsystems CMS, Am Friedensplatz 3, 68126 Mannheim, Germany ABSTRACT
New developments in STED Microscopy Arnold Giske*, Jochen Sieber, Hilmar Gugel, Marcus Dyba, Volker Seyfried, Dietmar Gnass Leica Microsystems CMS, Am Friedensplatz 3, 68126 Mannheim, Germany ABSTRACT
Bi177 - Lecture 13 Microscopy Outside the Box. Fluorescence Nanoscopy TIRF 4-pi STED STORM/PALM
 Bi177 - Lecture 13 Microscopy Outside the Box Fluorescence Nanoscopy TIRF 4-pi STED STORM/PALM The diffraction limit: Abbe s law The Problem Diffraction limit 100x larger than molecular scale! Green Fluorescent
Bi177 - Lecture 13 Microscopy Outside the Box Fluorescence Nanoscopy TIRF 4-pi STED STORM/PALM The diffraction limit: Abbe s law The Problem Diffraction limit 100x larger than molecular scale! Green Fluorescent
Syddansk Universitet. Pathways to optical STED microscopy
 Syddansk Universitet Pathways to optical STED microscopy Clausen, Mathias P.; Galiani, Silvia; Serna, Jorge Bernardino de la ; Fritzsche, Marco; Chojnaki, Jakub; Gehmlich, Katja; Lagerholm, B. Christoffer;
Syddansk Universitet Pathways to optical STED microscopy Clausen, Mathias P.; Galiani, Silvia; Serna, Jorge Bernardino de la ; Fritzsche, Marco; Chojnaki, Jakub; Gehmlich, Katja; Lagerholm, B. Christoffer;
Super-resolution Microscopy
 Semr oc kwhi t epaperser i es : 1. Introduction Super-resolution Microscopy Fluorescence microscopy has revolutionized the study of biological samples. Ever since the invention of fluorescence microscopy
Semr oc kwhi t epaperser i es : 1. Introduction Super-resolution Microscopy Fluorescence microscopy has revolutionized the study of biological samples. Ever since the invention of fluorescence microscopy
A Brief History of Light Microscopy And How It Transformed Biomedical Research
 A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
Sample region with fluorescent labeled molecules
 FLUORESCENCE IMAGING I. Fluorescence-imaging with diffraction limited spots The resolution in optical microscopy has been hampered by the smallest spot possible (~ λ/2) that can be achieved by conventional
FLUORESCENCE IMAGING I. Fluorescence-imaging with diffraction limited spots The resolution in optical microscopy has been hampered by the smallest spot possible (~ λ/2) that can be achieved by conventional
Q&A: Single-molecule localization microscopy for biological imaging
 Q U E S T I O N & ANSWER Q&A: Single-molecule localization microscopy for biological imaging Ann L McEvoy 1, Derek Greenfield 1,2,5, Mark Bates 3 and Jan Liphardt 1,2,4 * Open Access Why is it important
Q U E S T I O N & ANSWER Q&A: Single-molecule localization microscopy for biological imaging Ann L McEvoy 1, Derek Greenfield 1,2,5, Mark Bates 3 and Jan Liphardt 1,2,4 * Open Access Why is it important
Journal of Biotechnology
 Journal of Biotechnology 149 (2010) 243 251 Contents lists available at ScienceDirect Journal of Biotechnology journal homepage: www.elsevier.com/locate/jbiotec Fluorescence microscopy beyond the diffraction
Journal of Biotechnology 149 (2010) 243 251 Contents lists available at ScienceDirect Journal of Biotechnology journal homepage: www.elsevier.com/locate/jbiotec Fluorescence microscopy beyond the diffraction
PALM/STORM, BALM, STED
 PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
Tracking sub-microscopic protein organisation at the plasma membrane of live cells using triple-colour superresolution
 Tracking sub-microscopic protein organisation at the plasma membrane of live cells using triple-colour superresolution microscopy Jacob Piehler Division of Biophysics, University of Osnabrück, Germany
Tracking sub-microscopic protein organisation at the plasma membrane of live cells using triple-colour superresolution microscopy Jacob Piehler Division of Biophysics, University of Osnabrück, Germany
Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc.
 15/05/2012 Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc. Prof. Dr. Rainer Duden duden@bio.uni-luebeck.de 1 Using conventional light microscopy resolution is limited
15/05/2012 Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc. Prof. Dr. Rainer Duden duden@bio.uni-luebeck.de 1 Using conventional light microscopy resolution is limited
SUPER-RESOLUTION MICROSCOPY. Dr. Nathalie Garin
 SUPER-RESOLUTION MICROSCOPY Dr. Nathalie Garin Content Motivation for superresolution Superresolution, nanoscopy, : definition Structured Illumination Microscopy (SIM) Localization microscopy STimulated
SUPER-RESOLUTION MICROSCOPY Dr. Nathalie Garin Content Motivation for superresolution Superresolution, nanoscopy, : definition Structured Illumination Microscopy (SIM) Localization microscopy STimulated
Biophysical Journal Volume 107 October Super-resolution Microscopy Approaches for Live Cell Imaging
 Biophysical Journal Volume 107 October 2014 1777 1784 1777 Biophysical Review Super-resolution Microscopy Approaches for Live Cell Imaging Antoine G. Godin, 1,2 Brahim Lounis, 1,2 and Laurent Cognet 1,2,
Biophysical Journal Volume 107 October 2014 1777 1784 1777 Biophysical Review Super-resolution Microscopy Approaches for Live Cell Imaging Antoine G. Godin, 1,2 Brahim Lounis, 1,2 and Laurent Cognet 1,2,
Introduction to Computational Fluorescence Microscopy!
 Introduction to Computational Fluorescence Microscopy! EE367/CS448I: Computational Imaging and Display! stanford.edu/class/ee367! Lecture 13! Gordon Wetzstein! Stanford University! Midterm! Tuesday, Feb
Introduction to Computational Fluorescence Microscopy! EE367/CS448I: Computational Imaging and Display! stanford.edu/class/ee367! Lecture 13! Gordon Wetzstein! Stanford University! Midterm! Tuesday, Feb
Breaking the Diffraction Barrier: Super-Resolution Imaging of Cells
 Leading Edge Primer Breaking the Diffraction Barrier: Super-Resolution Imaging of Cells Bo Huang, 1 Hazen Babcock, 2 and Xiaowei Zhuang 2,3, * 1 Department of Pharmaceutical Chemistry and Department of
Leading Edge Primer Breaking the Diffraction Barrier: Super-Resolution Imaging of Cells Bo Huang, 1 Hazen Babcock, 2 and Xiaowei Zhuang 2,3, * 1 Department of Pharmaceutical Chemistry and Department of
Localization-Based Super-Resolution Light Microscopy
 Kristin A. Gabor, 1,2,3 Mudalige S. Gunewardene, 1 David Santucci, 4 and Samuel T. Hess 1,3, * 1 Department of Physics and Astronomy, 120 Bennett Hall, University of Maine, Orono, ME 04469 2 Department
Kristin A. Gabor, 1,2,3 Mudalige S. Gunewardene, 1 David Santucci, 4 and Samuel T. Hess 1,3, * 1 Department of Physics and Astronomy, 120 Bennett Hall, University of Maine, Orono, ME 04469 2 Department
Nanoscale measurement examples in biology: Sub-diffractive imaging & the fly brain challenge
 Nanoscale measurement examples in biology: Sub-diffractive imaging & the fly brain challenge Harald Hess, HHMI Janelia Farm Introduction to HHMI Janelia Farms Sample: Nerves, Worm Brains, Fly Brains, Rodent
Nanoscale measurement examples in biology: Sub-diffractive imaging & the fly brain challenge Harald Hess, HHMI Janelia Farm Introduction to HHMI Janelia Farms Sample: Nerves, Worm Brains, Fly Brains, Rodent
Confocal Microscopy & Imaging Technology. Yan Wu
 Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Abstract. Introduction
 Abstract The investigation of interactions between engineered nanostructures and biological systems is a key component in the assessment of potential environmental and health implications due to the increasing
Abstract The investigation of interactions between engineered nanostructures and biological systems is a key component in the assessment of potential environmental and health implications due to the increasing
Imaging Living Synapses at the Nanoscale by STED Microscopy
 The Journal of Neuroscience, July 14, 2010 30(28):9341 9346 9341 Toolbox Editor s Note: Toolboxes are intended to describe and evaluate methods that are becoming widely relevant to the neuroscience community
The Journal of Neuroscience, July 14, 2010 30(28):9341 9346 9341 Toolbox Editor s Note: Toolboxes are intended to describe and evaluate methods that are becoming widely relevant to the neuroscience community
8:00 pm David A. Agard, HHMI/University of California, San Francisco Welcome and introduction to the meeting
 Sunday, May 20 th 3:00 pm Check-in 6:00 pm Reception 7:00 pm Dinner 8:00 pm Session 1: Introduction 8:00 pm David A. Agard, HHMI/University of California, San Francisco Welcome and introduction to the
Sunday, May 20 th 3:00 pm Check-in 6:00 pm Reception 7:00 pm Dinner 8:00 pm Session 1: Introduction 8:00 pm David A. Agard, HHMI/University of California, San Francisco Welcome and introduction to the
Fast, three-dimensional super-resolution imaging of live cells
 Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
F* techniques: FRAP, FLIP, FRET, FLIM,
 F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
Localization Microscopy
 Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
Review of recent developments in stimulated emission depletion microscopy: applications on cell imaging
 Review of recent developments in stimulated emission depletion microscopy: applications on cell imaging Bhanu Neupane Frances S. Ligler Gufeng Wang Journal of Biomedical Optics 19(8), 080901 (August 2014)
Review of recent developments in stimulated emission depletion microscopy: applications on cell imaging Bhanu Neupane Frances S. Ligler Gufeng Wang Journal of Biomedical Optics 19(8), 080901 (August 2014)
Lasers for Microscopy: Major Trends
 Lasers for Microscopy: Major Trends Marco Arrigoni, Nigel Gallaher, Darryl McCoy, Volker Pfeufer and Matthias Schulze, Coherent Inc. Laser development for the microscopy market continues to be driven by
Lasers for Microscopy: Major Trends Marco Arrigoni, Nigel Gallaher, Darryl McCoy, Volker Pfeufer and Matthias Schulze, Coherent Inc. Laser development for the microscopy market continues to be driven by
Super-resolution Microscopy Approaches for Live Cell Imaging
 Super-resolution Microscopy Approaches for Live Cell Imaging Antoine Godin, Brahim Lounis, Laurent Cognet To cite this version: Antoine Godin, Brahim Lounis, Laurent Cognet. Super-resolution Microscopy
Super-resolution Microscopy Approaches for Live Cell Imaging Antoine Godin, Brahim Lounis, Laurent Cognet To cite this version: Antoine Godin, Brahim Lounis, Laurent Cognet. Super-resolution Microscopy
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers
 Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Journal of Molecular and Cellular Cardiology
 Journal of Molecular and Cellular Cardiology 58 (2013) 13 21 Contents lists available at SciVerse ScienceDirect Journal of Molecular and Cellular Cardiology journal homepage: www.elsevier.com/locate/yjmcc
Journal of Molecular and Cellular Cardiology 58 (2013) 13 21 Contents lists available at SciVerse ScienceDirect Journal of Molecular and Cellular Cardiology journal homepage: www.elsevier.com/locate/yjmcc
STED nanoscopy with mass-produced laser diodes
 STED nanoscopy with mass-produced laser diodes Susanne Schrof, 1,4 Thorsten Staudt, 1,4 Eva Rittweger, 1 Nina Wittenmayer, 3 Thomas Dresbach, 3 Johann Engelhardt, 1 and Stefan W. Hell 1,2,* 1 German Cancer
STED nanoscopy with mass-produced laser diodes Susanne Schrof, 1,4 Thorsten Staudt, 1,4 Eva Rittweger, 1 Nina Wittenmayer, 3 Thomas Dresbach, 3 Johann Engelhardt, 1 and Stefan W. Hell 1,2,* 1 German Cancer
In spite of its long history, optical
 Major Trends Laser development for the microscopy market continues to be driven by key trends in applications, which currently include superresolution techniques, multiphoton applications in optogenetics
Major Trends Laser development for the microscopy market continues to be driven by key trends in applications, which currently include superresolution techniques, multiphoton applications in optogenetics
The importance of the photon arrival times in STED microscopy
 he importance of the photon arrival times in SED microscopy Marco Castello a,b, Luca Lanzanó a, Iván Coto Hernández a,d, Christian Eggeling c, Alberto Diaspro a,d,e and Giuseppe Vicidomini a a Nanophysics,
he importance of the photon arrival times in SED microscopy Marco Castello a,b, Luca Lanzanó a, Iván Coto Hernández a,d, Christian Eggeling c, Alberto Diaspro a,d,e and Giuseppe Vicidomini a a Nanophysics,
Assembly of synapses by neuronal adhesion molecules: single molecule studies
 Assembly of synapses by neuronal adhesion molecules: single molecule studies Olivier Thoumine Interdisciplinary Institute of Neurosciences CNRS - University of Bordeaux Connectivity in the brain 300 nm
Assembly of synapses by neuronal adhesion molecules: single molecule studies Olivier Thoumine Interdisciplinary Institute of Neurosciences CNRS - University of Bordeaux Connectivity in the brain 300 nm
Introduction to N-STORM
 Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Size Estimation of Protein Clusters in the Nanometer Range by Using Spatially Modulated Illumination Microscopy
 FORMATEX 2007 A. Méndez-Vilas and J. Díaz (Eds.) Size Estimation of Protein Clusters in the Nanometer Range by Using Spatially Modulated Illumination Microscopy U. J. Birk 1,2, I. Upmann 1, D. Toomre 3,
FORMATEX 2007 A. Méndez-Vilas and J. Díaz (Eds.) Size Estimation of Protein Clusters in the Nanometer Range by Using Spatially Modulated Illumination Microscopy U. J. Birk 1,2, I. Upmann 1, D. Toomre 3,
Supporting Information
 Supporting Information Azido Push Pull Fluorogens Photoactivate to Produce Bright Fluorescent Labels Samuel J. Lord, Hsiao-lu D. Lee, Reichel Samuel, Ryan Weber, Na Liu, Nicholas R. Conley, Michael A.
Supporting Information Azido Push Pull Fluorogens Photoactivate to Produce Bright Fluorescent Labels Samuel J. Lord, Hsiao-lu D. Lee, Reichel Samuel, Ryan Weber, Na Liu, Nicholas R. Conley, Michael A.
Two-Photon Microscopy for Deep Tissue Imaging of Living Specimens
 for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
Two-photon excitation STED microscopy
 Two-photon excitation STED microscopy Gael Moneron and Stefan W. Hell* Department of NanoBiophotonics, Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, 37077 Göttingen, Germany *shell@gwdg.de
Two-photon excitation STED microscopy Gael Moneron and Stefan W. Hell* Department of NanoBiophotonics, Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, 37077 Göttingen, Germany *shell@gwdg.de
Fluorescence microscopy
 Fluorescence microscopy 1 Fluorescence microscopies basic fluorescence, fluorophores Deconvolution Confocal Two-photon/multi-photon 4Pi Light sheet Total internal reflection STED FRAP/FLIP/FCS FRET PALM/STORM/iPALM
Fluorescence microscopy 1 Fluorescence microscopies basic fluorescence, fluorophores Deconvolution Confocal Two-photon/multi-photon 4Pi Light sheet Total internal reflection STED FRAP/FLIP/FCS FRET PALM/STORM/iPALM
a) JOURNAL OF BIOLOGICAL CHEMISTRY b) PNAS c) NATURE
 a) JOURNAL OF BIOLOGICAL CHEMISTRY b) c) d) ........................ JOURNAL OF BIOLOGICAL CHEMISTRY MOLECULAR PHARMACOLOGY TRENDS IN PHARMACOLOGICAL S AMERICAN JOURNAL OF PHYSIOLOGY-HEART AND CIRCULATORY
a) JOURNAL OF BIOLOGICAL CHEMISTRY b) c) d) ........................ JOURNAL OF BIOLOGICAL CHEMISTRY MOLECULAR PHARMACOLOGY TRENDS IN PHARMACOLOGICAL S AMERICAN JOURNAL OF PHYSIOLOGY-HEART AND CIRCULATORY
Single-Particle Tracking Photoactivated Localization Microscopy for Mapping Single-Molecule Dynamics
 CHAPTER FIVE Single-Particle Tracking Photoactivated Localization Microscopy for Mapping Single-Molecule Dynamics Suliana Manley,* Jennifer M. Gillette, and Jennifer Lippincott-Schwartz Contents 1. Introduction
CHAPTER FIVE Single-Particle Tracking Photoactivated Localization Microscopy for Mapping Single-Molecule Dynamics Suliana Manley,* Jennifer M. Gillette, and Jennifer Lippincott-Schwartz Contents 1. Introduction
Super-Resolution Localization Microscopy
 APPLICATION NOTE Super-Resolution Localization Microscopy Light microscopy techniques have been vital to our understanding of biological structures and systems since their invention in the late 16 th Century.
APPLICATION NOTE Super-Resolution Localization Microscopy Light microscopy techniques have been vital to our understanding of biological structures and systems since their invention in the late 16 th Century.
Next-generation optical microscopy
 Next-generation optical microscopy Rahul Roy* Department of Chemical Engineering, and Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012, India A new breed of microscopy techniques
Next-generation optical microscopy Rahul Roy* Department of Chemical Engineering, and Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012, India A new breed of microscopy techniques
Measure of surface protein mobility with u-paint technique
 Measure of surface protein mobility with u-paint technique How dynamic image can solve the situation? Random distribution or cluster? Why live super-resolution microscopy can solve the situation With mobility
Measure of surface protein mobility with u-paint technique How dynamic image can solve the situation? Random distribution or cluster? Why live super-resolution microscopy can solve the situation With mobility
Far-Field Optical Microscopy Based on Stimulated Emission Depletion
 University of South Carolina Scholar Commons Theses and Dissertations 2017 Far-Field Optical Microscopy Based on Stimulated Emission Depletion Yunxia Wang University of South Carolina Follow this and additional
University of South Carolina Scholar Commons Theses and Dissertations 2017 Far-Field Optical Microscopy Based on Stimulated Emission Depletion Yunxia Wang University of South Carolina Follow this and additional
Super Resolution Imaging Solution Provider. Imaging Future
 Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope
 High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
Nature Neuroscience: doi: /nn Supplementary Figure 1. Comprehensive opto-mechanical design of the dual-axis microscope.
 Supplementary Figure 1 Comprehensive opto-mechanical design of the dual-axis microscope. (a) The complete microscope is shown to scale. The laser beam is depicted in light red. (b) A close-up view of the
Supplementary Figure 1 Comprehensive opto-mechanical design of the dual-axis microscope. (a) The complete microscope is shown to scale. The laser beam is depicted in light red. (b) A close-up view of the
Imagerie et spectroscopie de fluorescence par excitation non radiative
 Imagerie et spectroscopie de fluorescence par excitation non radiative comment s affranchir de la limite de diffraction Rodolphe Jaffiol, Cyrille Vézy, Marcelina Cardoso Dos Santos LNIO, UTT, Troyes NanoBioPhotonics
Imagerie et spectroscopie de fluorescence par excitation non radiative comment s affranchir de la limite de diffraction Rodolphe Jaffiol, Cyrille Vézy, Marcelina Cardoso Dos Santos LNIO, UTT, Troyes NanoBioPhotonics
Symposium 20 years of nano-optics April 6th, 2004 Auditorium, Institute of Physics, St.Johanns-Ring 25
 Symposium 20 years of nano-optics April 6th, 2004 Auditorium, Institute of Physics, St.Johanns-Ring 25 9:30 9:45 Coffee and Gipfeli 9:45 10:00 Welcome address and introduction B. Hecht Uni Basel H.-J.
Symposium 20 years of nano-optics April 6th, 2004 Auditorium, Institute of Physics, St.Johanns-Ring 25 9:30 9:45 Coffee and Gipfeli 9:45 10:00 Welcome address and introduction B. Hecht Uni Basel H.-J.
SUPER-RESOLVED FLUORESCENCE MICROSCOPY
 8 OCTOBER 2014 Scientific Background on the Nobel Prize in Chemistry 2014 SUPER-RESOLVED FLUORESCENCE MICROSCOPY THE ROYAL SWEDISH ACADEMY OF SCIENCES has as its aim to promote the sciences and strengthen
8 OCTOBER 2014 Scientific Background on the Nobel Prize in Chemistry 2014 SUPER-RESOLVED FLUORESCENCE MICROSCOPY THE ROYAL SWEDISH ACADEMY OF SCIENCES has as its aim to promote the sciences and strengthen
Quantification of fluid temperature field using fluorescent anisotropy
 Quantification of fluid temperature field using fluorescent anisotropy Yuta Shugyo 1, Takuya Aida 1, Masahiro Motosuke 1,2,* 1: Dept. of Mechanical Engineering, Tokyo University of Science, Japan 2: Research
Quantification of fluid temperature field using fluorescent anisotropy Yuta Shugyo 1, Takuya Aida 1, Masahiro Motosuke 1,2,* 1: Dept. of Mechanical Engineering, Tokyo University of Science, Japan 2: Research
Supplementary Table 1. Components of an FCS setup (1PE and 2PE)
 Supplementary Table 1. Components of an FCS setup (1PE and 2PE) Component and function Laser source Excitation of fluorophores Microscope with xy-translation stage mounted on vibration isolated optical
Supplementary Table 1. Components of an FCS setup (1PE and 2PE) Component and function Laser source Excitation of fluorophores Microscope with xy-translation stage mounted on vibration isolated optical
Confocal Microscopes. Evolution of Imaging
 Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
Far-field fluorescence microscopy beyond the diffraction limit: Fluorescence imaging with ultrahigh resolution
 Far-field fluorescence microscopy beyond the diffraction limit: Fluorescence imaging with ultrahigh resolution James H. Rice School of Chemical Sciences and Pharmacy, University of East Anglia, Norwich
Far-field fluorescence microscopy beyond the diffraction limit: Fluorescence imaging with ultrahigh resolution James H. Rice School of Chemical Sciences and Pharmacy, University of East Anglia, Norwich
To study the fine details of biological structures with
 pubs.acs.org/nanolett Terms of Use CC-BY Super-Resolution Microscopy Using Standard Fluorescent Proteins in Intact Cells under Cryo-Conditions Rainer Kaufmann,, Pascale Schellenberger, Elena Seiradake,
pubs.acs.org/nanolett Terms of Use CC-BY Super-Resolution Microscopy Using Standard Fluorescent Proteins in Intact Cells under Cryo-Conditions Rainer Kaufmann,, Pascale Schellenberger, Elena Seiradake,
Vertico-SMI /SPDMphymod: Next Level of Super-Resolution Fluorescence Microscopy
 Work in your familiar GFP/YFP/RFP system from the first experiment to the nanoimage Bwcon business award winner: Inventor Prof Christoph Cremer Vertico-SMI /SPDMphymod: Next Level of Super-Resolution Fluorescence
Work in your familiar GFP/YFP/RFP system from the first experiment to the nanoimage Bwcon business award winner: Inventor Prof Christoph Cremer Vertico-SMI /SPDMphymod: Next Level of Super-Resolution Fluorescence
SUPER-RESOLUTION MICROSCOPY
 University of Groningen Faculty of Mathematics and Natural Sciences Zernike Institute for Advanced Materials Nanoscience Research Paper Mykhailo Gerasymenko Top Master in Nanosience m.gerasymenko@student.rug.nl
University of Groningen Faculty of Mathematics and Natural Sciences Zernike Institute for Advanced Materials Nanoscience Research Paper Mykhailo Gerasymenko Top Master in Nanosience m.gerasymenko@student.rug.nl
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity
 Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
DevelopmentintheSTORM
 DevelopmentintheSTORM Daichi Kamiyama 1, * and Bo Huang 1,2, * 1 Department of Pharmaceutical Chemistry 2 Department of Biochemistry and Biophysics University of California, San Francisco, San Francisco,
DevelopmentintheSTORM Daichi Kamiyama 1, * and Bo Huang 1,2, * 1 Department of Pharmaceutical Chemistry 2 Department of Biochemistry and Biophysics University of California, San Francisco, San Francisco,
Stochastic Optical Reconstruction Microscopy (STORM): A Method for Superresolution Fluorescence Imaging
 Topic Introduction Stochastic Optical Reconstruction Microscopy (STORM): A Method for Superresolution Fluorescence Imaging Mark Bates, Sara A. Jones, and Xiaowei Zhuang The relatively low spatial resolution
Topic Introduction Stochastic Optical Reconstruction Microscopy (STORM): A Method for Superresolution Fluorescence Imaging Mark Bates, Sara A. Jones, and Xiaowei Zhuang The relatively low spatial resolution
Fluorescence Nanoscopy 高甫仁 ) Institute of Biophotonics, National Yang Ming University. Outline
 Fluorescence Nanoscopy 高甫仁 ) Fu-Jen Kao ( 高甫仁 Institute of Biophotonics, National Yang Ming University Outline The Abbe s (diffraction) limit and nanoscopy Fundamentals and opportunities of FLIM/FRET Visualizing
Fluorescence Nanoscopy 高甫仁 ) Fu-Jen Kao ( 高甫仁 Institute of Biophotonics, National Yang Ming University Outline The Abbe s (diffraction) limit and nanoscopy Fundamentals and opportunities of FLIM/FRET Visualizing
HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING. Marc Verhaegen
 HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING Marc Verhaegen CMCS, MONTREAL, MAY 11 th, 2017 OVERVIEW Hyperspectral Imaging Multiplex Biological Imaging Multiplex Single Particle
HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING Marc Verhaegen CMCS, MONTREAL, MAY 11 th, 2017 OVERVIEW Hyperspectral Imaging Multiplex Biological Imaging Multiplex Single Particle
Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility
 Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility Sabrina Campelo, Dr. Andrew Harken Outline Motivation Fluorescence Microscopy -Multiphoton Imaging
Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility Sabrina Campelo, Dr. Andrew Harken Outline Motivation Fluorescence Microscopy -Multiphoton Imaging
Microscopy. CS/CME/BioE/Biophys/BMI 279 Nov. 2, 2017 Ron Dror
 Microscopy CS/CME/BioE/Biophys/BMI 279 Nov. 2, 2017 Ron Dror 1 Outline Microscopy: the basics Fluorescence microscopy Resolution limits The diffraction limit Beating the diffraction limit 2 Microscopy:
Microscopy CS/CME/BioE/Biophys/BMI 279 Nov. 2, 2017 Ron Dror 1 Outline Microscopy: the basics Fluorescence microscopy Resolution limits The diffraction limit Beating the diffraction limit 2 Microscopy:
Visualizing Cells Molecular Biology of the Cell - Chapter 9
 Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Rice/TCU REU on Computational Neuroscience. Fundamentals of Molecular Imaging
 Rice/TCU REU on Computational Neuroscience Fundamentals of Molecular Imaging June 2, 2009 Neal Waxham 713-500-5621 m.n.waxham@uth.tmc.edu Objectives Introduction to resolution in light microscopy Brief
Rice/TCU REU on Computational Neuroscience Fundamentals of Molecular Imaging June 2, 2009 Neal Waxham 713-500-5621 m.n.waxham@uth.tmc.edu Objectives Introduction to resolution in light microscopy Brief
HHS Public Access Author manuscript Phys Rev Lett. Author manuscript; available in PMC 2014 March 18.
 Photo-imprint Photoacoustic Microscopy for Three-dimensional Label-free Sub-diffraction Imaging Junjie Yao, Lidai Wang, Chiye Li, Chi Zhang, and Lihong V. Wang * Optical Imaging Laboratory, Department
Photo-imprint Photoacoustic Microscopy for Three-dimensional Label-free Sub-diffraction Imaging Junjie Yao, Lidai Wang, Chiye Li, Chi Zhang, and Lihong V. Wang * Optical Imaging Laboratory, Department
Genetically targeted all-optical electrophysiology with a transgenic Credependent
 Genetically targeted all-optical electrophysiology with a transgenic Credependent Optopatch mouse Short title: Transgenic Optopatch mouse Shan Lou 1, Yoav Adam 1, Eli N. Weinstein 1,4, Erika Williams 2,
Genetically targeted all-optical electrophysiology with a transgenic Credependent Optopatch mouse Short title: Transgenic Optopatch mouse Shan Lou 1, Yoav Adam 1, Eli N. Weinstein 1,4, Erika Williams 2,
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
A photoprotection strategy for microsecond-resolution single-molecule fluorescence spectroscopy
 Nature Methods A photoprotection strategy for microsecond-resolution single-molecule fluorescence spectroscopy Luis A Campos, Jianwei Liu, Xiang Wang, Ravishankar Ramanathan, Douglas S English & Victor
Nature Methods A photoprotection strategy for microsecond-resolution single-molecule fluorescence spectroscopy Luis A Campos, Jianwei Liu, Xiang Wang, Ravishankar Ramanathan, Douglas S English & Victor
Single cell molecular profiling using Quantum Dots. Technical Journal Club Rahel Gerosa
 Single cell molecular profiling using Quantum Dots Technical Journal Club 01.10.2013 Rahel Gerosa Molecular Profiling Powerful technique to study complex molecular networks underlying physiological and
Single cell molecular profiling using Quantum Dots Technical Journal Club 01.10.2013 Rahel Gerosa Molecular Profiling Powerful technique to study complex molecular networks underlying physiological and
Absorption of an electromagnetic wave
 In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
FLIM Fluorescence Lifetime IMaging
 FLIM Fluorescence Lifetime IMaging Fluorescence lifetime t I(t) = F0 exp( ) τ 1 τ = k f + k nr k nr = k IC + k ISC + k bl Batiaens et al, Trends in Cell Biology, 1999 τ τ = fluorescence lifetime (~ns to
FLIM Fluorescence Lifetime IMaging Fluorescence lifetime t I(t) = F0 exp( ) τ 1 τ = k f + k nr k nr = k IC + k ISC + k bl Batiaens et al, Trends in Cell Biology, 1999 τ τ = fluorescence lifetime (~ns to
F luorescence imaging of live cells plays a crucial role in the study of biological processes at the cellular and
 SUBJECT AREAS: SUPER-RESOLUTION MICROSCOPY WIDE-FIELD FLUORESCENCE MICROSCOPY BIOPHOTONICS SINGLE-MOLECULE BIOPHYSICS Received 6 December 2012 Accepted 4 January 2013 Published 4 February 2013 Correspondence
SUBJECT AREAS: SUPER-RESOLUTION MICROSCOPY WIDE-FIELD FLUORESCENCE MICROSCOPY BIOPHOTONICS SINGLE-MOLECULE BIOPHYSICS Received 6 December 2012 Accepted 4 January 2013 Published 4 February 2013 Correspondence
Supplementary Information
 Supplementary Information STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA Iddo Heller, Gerrit Sitters, Onno D. Broekmans, Géraldine Farge, Carolin Menges,
Supplementary Information STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA Iddo Heller, Gerrit Sitters, Onno D. Broekmans, Géraldine Farge, Carolin Menges,
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Enlightening G-protein-coupled receptors on the plasma membrane using super-resolution photoactivated localization microscopy
 G-Protein-Coupled Receptors: from Structural Insights to Functional Mechanisms 191 Enlightening G-protein-coupled receptors on the plasma membrane using super-resolution photoactivated localization microscopy
G-Protein-Coupled Receptors: from Structural Insights to Functional Mechanisms 191 Enlightening G-protein-coupled receptors on the plasma membrane using super-resolution photoactivated localization microscopy
CENTER FOR BRAIN EXPERIMENT
 CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
PEER REVIEW FILE. Reviewers' comments: Reviewer #1 (Remarks to the Author):
 PEER REVIEW FILE Reviewers' comments: Reviewer #1 (Remarks to the Author): General In its beginnings in the mid 1990s, localization microscopy based on optical isolation of fluorescent point targets was
PEER REVIEW FILE Reviewers' comments: Reviewer #1 (Remarks to the Author): General In its beginnings in the mid 1990s, localization microscopy based on optical isolation of fluorescent point targets was
Molecular BioSystems, 3 (11):
 Provided by the author(s) and University College Dublin Library in accordance with publisher policies. Please cite the published version when available. Title Beyond the diffraction limit : far-field fluorescence
Provided by the author(s) and University College Dublin Library in accordance with publisher policies. Please cite the published version when available. Title Beyond the diffraction limit : far-field fluorescence
Biophotonics?? Biophotonics. technology in biomedical engineering. Advantages of the lightwave
 Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
Multiphoton Microscopy: Seeing deeper and clearer
 Multiphoton Microscopy: Seeing deeper and clearer Since the invention of simple microscope by Leuwenhoek and Hooke in the 17th century, different types of light microscopy techniques (such as phase contrast,
Multiphoton Microscopy: Seeing deeper and clearer Since the invention of simple microscope by Leuwenhoek and Hooke in the 17th century, different types of light microscopy techniques (such as phase contrast,
The new LSM 700 from Carl Zeiss
 The new LSM 00 from Carl Zeiss Olaf Selchow, Bernhard Goetze To cite this version: Olaf Selchow, Bernhard Goetze. The new LSM 00 from Carl Zeiss. Biotechnology Journal, Wiley- VCH Verlag, 0, (), pp.. .
The new LSM 00 from Carl Zeiss Olaf Selchow, Bernhard Goetze To cite this version: Olaf Selchow, Bernhard Goetze. The new LSM 00 from Carl Zeiss. Biotechnology Journal, Wiley- VCH Verlag, 0, (), pp.. .
STED microscopy super-resolution bio-imaging utilizing a stimulated emission depletion
 Microscopy Advance Access published July 6, 2015 Microscopy, 2015, 1 10 doi: 10.1093/jmicro/dfv036 Review STED microscopy super-resolution bio-imaging utilizing a stimulated emission depletion Kohei Otomo
Microscopy Advance Access published July 6, 2015 Microscopy, 2015, 1 10 doi: 10.1093/jmicro/dfv036 Review STED microscopy super-resolution bio-imaging utilizing a stimulated emission depletion Kohei Otomo
Microscopy from Carl Zeiss. DirectFRAP. News from the Cell. The New Class of Laser Manipulation for the Analysis of Cell Dynamics
 Microscopy from Carl Zeiss DirectFRAP News from the Cell The New Class of Laser Manipulation for the Analysis of Cell Dynamics DirectFRAP. New Insights into Cell Dynamics. Fluorescence breaks new ground:
Microscopy from Carl Zeiss DirectFRAP News from the Cell The New Class of Laser Manipulation for the Analysis of Cell Dynamics DirectFRAP. New Insights into Cell Dynamics. Fluorescence breaks new ground:
Achieving lambda/10 Resolution CW STED Nanoscopy with a Ti:Sapphire Oscillator
 Achieving lambda/10 Resolution CW STED Nanoscopy with a Ti:Sapphire Oscillator Yujia Liu, Shanghai Jiao Tong University Yichen Ding, Peking University Eric Alonas, Georgia Institute of Technology Wenli
Achieving lambda/10 Resolution CW STED Nanoscopy with a Ti:Sapphire Oscillator Yujia Liu, Shanghai Jiao Tong University Yichen Ding, Peking University Eric Alonas, Georgia Institute of Technology Wenli
MicroTime 200 STED. Super-resolution add-on for the confocal time-resolved microscopy platform
 MicroTime 200 STED Super-resolution add-on for the confocal time-resolved microscopy platform confocal STED 2 Vision The MicroTime 200... The MicroTime 200 is a high-end confocal fluorescence lifetime
MicroTime 200 STED Super-resolution add-on for the confocal time-resolved microscopy platform confocal STED 2 Vision The MicroTime 200... The MicroTime 200 is a high-end confocal fluorescence lifetime
Accelerated super-resolution imaging with FRET-PAINT
 Lee et al. Molecular Brain (2017) 10:63 DOI 10.1186/s13041-017-0344-5 RESEARCH Accelerated super-resolution imaging with FRET-PAINT Jongjin Lee 1,2, Sangjun Park 1,2, Wooyoung Kang 1,2 and Sungchul Hohng
Lee et al. Molecular Brain (2017) 10:63 DOI 10.1186/s13041-017-0344-5 RESEARCH Accelerated super-resolution imaging with FRET-PAINT Jongjin Lee 1,2, Sangjun Park 1,2, Wooyoung Kang 1,2 and Sungchul Hohng
Workshop advanced light microscopy
 Workshop advanced light microscopy Multi-mode confocal laser scanning microscope Jan Willem Borst Laboratory of Biochemistry Biomolecular Networks www.bic.wur.nl MicroSpectroscopy Centre Wageningen Microspectroscopy
Workshop advanced light microscopy Multi-mode confocal laser scanning microscope Jan Willem Borst Laboratory of Biochemistry Biomolecular Networks www.bic.wur.nl MicroSpectroscopy Centre Wageningen Microspectroscopy
Lab 5: Optical trapping and single molecule fluorescence
 Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm
 Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm Yina Wang, 1,2,5 Joerg Schnitzbauer, 3,5 Zhe Hu, 1,2,5 Xueming Li, 4 Yifan Cheng,
Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm Yina Wang, 1,2,5 Joerg Schnitzbauer, 3,5 Zhe Hu, 1,2,5 Xueming Li, 4 Yifan Cheng,
D e c N o. 2 8
 D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging
 Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging Graham T Dempsey 1,6, Joshua C Vaughan 2,3,6, Kok Hao Chen 3,6, Mark Bates 4 & Xiaowei Zhuang 2,3,5 211
Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging Graham T Dempsey 1,6, Joshua C Vaughan 2,3,6, Kok Hao Chen 3,6, Mark Bates 4 & Xiaowei Zhuang 2,3,5 211
