DevelopmentintheSTORM
|
|
|
- Lilian Richardson
- 6 years ago
- Views:
Transcription
1 DevelopmentintheSTORM Daichi Kamiyama 1, * and Bo Huang 1,2, * 1 Department of Pharmaceutical Chemistry 2 Department of Biochemistry and Biophysics University of California, San Francisco, San Francisco, CA 94158, USA *Correspondence: daichi.kamiyama@ucsf.edu (D.K.), bo.huang@ucsf.edu (B.H.) The recent invention of superresolution microscopy has brought up much excitement in the biological research community. Here, we focus on stochastic optical reconstruction microscopy/photoactivated localization microscopy (STORM/PALM) to discuss the challenges in applying superresolution microscopy to the study of developmental biology, including tissue imaging, sample preparation artifacts, and image interpretation. We also summarize new opportunities that superresolution microscopy could bring to the field of developmental biology. Introduction In the past several years, the emergence of superresolution microscopy has extended the resolution of fluorescence microscopy by more than an order of magnitude, almost reaching the scale of macromolecules (Hell, 2007, 2009; Huang et al., 2009, 2010). This new resolving power has immediately attracted the attention from biologists wondering whether many unanswered mysteries can now be clarified and old dogma be revisited. Indeed, the focus of the superresolution microscopy field has recently shifted from technological advancement to biological applications, with a number of new discoveries already made in cell biology (Kanchanawong et al., 2010; Wu et al., 2010), neurobiology (Beaudoin et al., 2012; Dani et al., 2010; Frost et al., 2010), and microbiology (Wang et al., 2011). What can superresolution microscopy do for developmental biology then? Are current technologies adequate to perform imaging in the context of a complex organism? What are the challenges and opportunities? Superresolution microscopy refers to a collection of new fluorescence microscopy methods that offer spatial resolutions far beyond the classical limit set by the diffraction of light. All of them achieve diffraction-unlimited spatial resolution by modulating close-by fluorescent molecules into different states, thus distinguishing their fluorescence signal. One approach to achieve this distinction is to spatially modulate the illumination light. The best known techniques using this approach are stimulated emission depletion (STED) microscopy (Hell and Wichmann, 1994; Klar and Hell, 1999) and structured illumination microscopy (SIM) (Gustafsson, 2000, 2005). The other approach is based on stochastically switching individual fluorescent molecules between a fluorescent and a dark state, which was independently invented under the names of stochastic optical reconstruction microscopy (STORM) (Rust et al., 2006), photoactivated localization microscopy (PALM) (Betzig et al., 2006), and fluorescence photoactivation localization microscopy (FPALM) (Hess et al., 2006). This approach collects a series of fluorescent images, each containing a sparse subset of fluorophores (either fluorescent proteins or organic dyes) activated into the fluorescent state. A superresolution image is then reconstructed by determining the positions of individual activated fluorophores. Later implementations and improvements of this approach have added in more names, such as PALMIRA (Egner et al., 2007), dstorm (Heilemann et al., 2008), and GSDIM (Fölling et al., 2008). Some new developments even avoid the use of photoswitching (Burnette et al., 2011; Sharonov and Hochstrasser, 2006) and single-molecule localization (Dertinger et al., 2009; Mukamel et al., 2012; Zhu et al., 2012). Nevertheless, all these methods share the same fundamental principle, instrumentation, and, in most cases, the analysis procedure. Therefore, here for simplicity, we refer to this single-molecule approach of superresolution microscopy techniques by the two best known names as STORM/PALM. The optical configuration of a STORM/PALM microscopy is almost identical to a common total internal reflection fluorescence (TIRF) microscope. This hardware simplicity, its relatively low cost, and the high spatial resolution it can achieve make STORM/PALM particularly popular among labs who would like to join in as either developers or users of superresolution microscopy techniques. However, despite being relatively easy to set up, obtaining perfect STORM/PALM images in real applications is not necessarily an easy task. Here, we discuss the challenges and caveats when applying STORM/PALM to the study of developmental biology. We then provide a brief survey of opportunities in developmental biology where STORM/ PALM can make unique contributions. Although we only discuss STORM/PALM here, we note that STED microscopy and SIM are both powerful techniques that often see challenges and opportunities similar to those of STORM/PALM in biological applications. Superresolution at a Depth STORM/PALM has been extremely successful in producing beautiful images of subcellular structures, including threedimensional (3D) (Huang et al., 2008a, 2008b; Juette et al., 2008), multicolor (Bates et al., 2007; Bossi et al., 2008; Dedecker et al., 2012; Shroff et al., 2007) and live (Hess et al., 2007; Manley et al., 2008; Shroff et al., 2008) imaging of structures ranging from the plasma membrane to inside the nucleus. Nevertheless, except for a few cases, most of these achievements were done in cultured cells. On the contrary, the study of developmental biology routinely requires imaging in the tissue context, sometimes even long-term observation in living organisms. Many of Developmental Cell 23, December 11, 2012 ª2012 Elsevier Inc. 1103
2 Figure 1. STORM/PALM Imaging in Tissue Samples (A) Synapses in mouse brain cortex showing immunostained presynaptic scaffolding protein Bassoon and postsynaptic density protein Homer1 imaged in cryostat sections (Beaudoin et al., 2012). (B) Nuclei of human mammary MCF10A cell spheroids. Cells express histone H2B fused to photoactivatable fluorescent protein PAmCherry. Images were recorded at a depth of 100 mm. Adapted from Cella Zanacchi et al. (2011) with permission from Macmillan Publishers Ltd. these imaging tasks are challenging even for conventional fluorescence microscopy at diffraction-limited resolution. Understandably, superresolution microscopy in the tissue context could face much more difficulties. For STORM/PALM, the major challenge in tissue imaging is the requirement for high sensitivity to detect individual fluorophores. The signal from one fluorescent molecule is very weak. Typical photoactivatable fluorescent proteins allow fewer than 1,000 photons detected before photobleaching (Lippincott- Schwartz and Patterson, 2009). Organic dyes can be brighter, but the detected photon number is still smaller than 5,000 in most cases (Dempsey et al., 2011). With the development of high numerical aperture objectives and single-photon-sensitive cameras, single-molecule imaging has been made relatively straightforward for in vitro systems and in fixed cells. Nevertheless, detecting one fluorescent molecule in a large tissue volume still has two obstacles: signal loss due to tissue scattering and tissue-induced optical aberrations and the high background from autofluorescence and out-of-focus fluorophores. Although these two issues affect all fluorescence microscopy methods, conventional or superresolution, STORM/PALM particularly suffers because the resulting loss of signal-tonoise ratio directly translates into worse precision in determining molecule positions (i.e., the optical resolution; Thompson et al., 2002). For STORM/PALM in cultured cells, a common trick to reduce out-of-focus fluorescence background is to use an illumination scheme similar to TIRF microscopy but with the incident angle slightly smaller than the critical angle (Tokunaga et al., 2008). In this configuration, the excitation light is restricted to a depth within several micrometers from the coverslip, efficiently illuminating the cell but not the mounting media above. The wide use of this scheme has created the misunderstanding that STORM/PALM only works with thin samples under TIRF illumination. Very much on the contrary, the same strategy can be used to image tissue sections, as long as the structure of interest is within a few micrometers from the section surface. For example, imaging in mouse brain sections has allowed the characterization of the protein organization of synapses, as well as development-associated changes in the density, morphology, and receptor composition of synapses (Figure 1A) (Beaudoin et al., 2012; Dani et al., 2010). To image deeper into the tissue, many fluorescence microscopy techniques have been developed in the past years, including two-photon microscopy (Denk et al., 1990) and selective-plane illumination microscopy (SPIM) (Huisken et al., 2004) for optical sectioning, as well as the use of adaptive optics to correct for tissue-induced aberrations (Girkin et al., 2009). As the field of deep tissue imaging continues to advance rapidly, we have now started to see the application of these techniques in STORM/PALM. Two-photon absorption can restrict fluorophore activation to a thin plane (Vaziri et al., 2008), thus reducing the out-of-focus background and allowing imaging into a depth of several tens of micrometers (York et al., 2011). Using SPIM to confine both activation and excitation, superresolution images have been acquired at 100 mm depth into mammary cell spheroid (Figure 1B) (Cella Zanacchi et al., 2011). Resolution improvement at this depth and beyond is more limited, though, due to tissue scattering and aberrations. We expect that the use of adaptive optics (Izeddin et al., 2012) and/or new tissue clearing reagents (Hama et al., 2011) could substantially improve the use of superresolution microscopy in deep tissue imaging. Alternatively, combing optical microscopy with serial physical sectioning could image through a large volume of tissue sample without the complexities in optical sectioning methods (Micheva and Smith, 2007; Nanguneri et al., 2012). Superresolved Artifacts For STORM/PALM, the precision to determine the position of a fluorescent molecule can routinely reach 10 nm in standard deviation, or 25 nm in full-width at half-maximum, with some of the best numbers smaller than 5 nm in standard deviation (Aquino et al., 2011; Shtengel et al., 2009; Xu et al., 2012). These values are at the same scale as the size of protein molecules. Therefore, they can be smaller than the distance between adjacent fluorophores that label the sample structure. This discrepancy does not prevent us from studying the properties of individual molecules, for example, when trying to observe the trafficking of a low-copy-number membrane protein. However, even by labeling 100% of this membrane protein, the molecule positions measured by STORM/PALM are insufficient to describe the detailed membrane morphology due to the low density (Figure 2A). In other words, the effective resolution in visualizing a continuous structure is also limited by the density of fluorescent labels. A simplified description of this relationship is the Nyquist sampling theorem: The distance between adjacent 1104 Developmental Cell 23, December 11, 2012 ª2012 Elsevier Inc.
3 limits the temporal resolution of live imaging. Typically, to avoid overlap of single-molecule images, which hinders singlemolecule localization, the average density of activated fluorophores is controlled to be no higher than 0.5 per mm 2. Under this condition, 2,000 camera frames give a 32 nm average point-to-point distance if all points are homogeneously distributed. At a camera frame rate of 60 frames per second, this corresponds to a 33 s recording time to support a 64 nm spatial resolution. In practice, the effective spatial resolution could be substantially better depending on the shape of the structure. As an example, having all points in a 1 mm 2 area concentrated in one clathrin-coated pit (170 nm diameter) would lead to a density-limited resolution of about 10 nm. The temporal resolution can be improved using a faster camera (Jones et al., 2011) or algorithms that can identify single molecules even when their images start to overlap (Holden et al., 2011; Huang et al., 2011; Quan et al., 2011; Zhu et al., 2012). For fixed-sample imaging, on the other hand, the acquisition time can be arbitrarily long in order to sample through all fluorophores. However, inadequate labeling still causes the most commonly observed artifact in superresolution images: a clustered appearance. This problem can result from several possibilities: Figure 2. Clustering Artifacts in STORM/PALM (A) Three-dimensional superresolution imaging of Golgi protein Giantin in a B-SC-1 cell reveals the overall morphology of Golgi stacks (left), but the low density of Giantin makes the superresolution image discontinuous (right). With this low labeling density, judging whether some structures are connected can be difficult (arrows). (B) Superresolution images of immunostained microtubules in Drosophila S2 cells, comparing good fixation (left) and inadequate fixation (right). (C) Zoomed-in view of the boxed region in the microtubule image. Arrows point to clusters generated by individual antibody molecules either nonspecifically bound to the sample or bound to tubulin monomers not incorporated into microtubules. (D) Blinking of fluorescent protein meos2 causes the nonclustering SrcN15 to appear clustered on the plasma membrane. Adapted from Annibale et al. (2011b) under the Creative Commons Attribution License ( plosone.org/static/license.action). fluorophores needs to be smaller than one half of the feature size (Shroff et al., 2008), whereas a rigorous theory (Fitzgerald et al., 2012) is yet to be established to account for the sample shape and the randomness of probe labeling. In live-sample STORM/PALM, a longer acquisition time for each time step will allow the collection of more single-molecule activation events, thus leading to a higher localization point density and, correspondingly, a better point-density-limited spatial resolution. Therefore, the spatial resolution requirement (1) Imperfect sample fixation and permeabilization. The high spatial resolution of STORM/PALM can easily reveal any disruption of the ultrastructures during sample preparation. For example, immunostained microtubules are widely used to demonstrate the resolution of superresolution microscopy, but it is highly susceptible to depolymerization during fixation, which creates microtubules that appear to be broken (Figure 2B). Crosslinking and permeabilization may also cause the clustering of membrane proteins. To solve these problems, more electronmicroscopy-like sample fixation protocols or live imaging would be necessary. (2) Low labeling efficiency. In the crowded cellular environment, the accessibility to the labeling target by fluorescent probes can restrict the labeling efficiency and, hence, the ability to resolve small structures. Antigen retrieval sometimes helps in improving antibody accessibility in dense structures such as the postsynaptic density (Beaudoin et al., 2012), albeit with the risk of ultrastructure disruption. Smaller probes, such as nanobodies (Ries et al., 2012), enzymatic tags (Klein et al., 2011; Wombacher et al., 2010), and small molecules (e.g., fluorescently labeled phalloidin for actin [Xu et al., 2012] and a wide variety of organelle-specific dyes [Shim et al., 2012]), often have higher labeling efficiency compared to full-size antibodies. Labeling efficiency by fluorescent protein fusion to the target protein can be very high but is still limited by the existence of unlabeled endogenous protein, overexpression artifacts, and the fact that not all fluorescent protein molecules will properly mature and be detected. Therefore, new approaches that can specifically and efficiently label cellular targets with bright fluorophores are much desired. (3) Clustering of the fluorescent probe. In conventional fluorescence microscopy, it is a common practice to Developmental Cell 23, December 11, 2012 ª2012 Elsevier Inc. 1105
4 amplify the fluorescence signal by labeling the antibodies with multiple dyes per antibody molecule, by indirect immunofluorescence staining, and by tyramide signal amplification (TSA) and other peroxidase-based amplification techniques. These methods add many dye molecules to one detected target, thus increasing the fluorescence signal and compensating the low labeling efficiency. However, these practices give no signal increase in STORM/PALM because it always detects individual molecules. In fact, they not only impair the resolution because they increase the effective size of the probe (Huang et al., 2008b; Ries et al., 2012) but also exaggerate the clustering problem because it turns a single probe into many fluorophores clustered together (Figures 2A and 2B). Actually, the aforementioned issue exists in all fluorescence microscopy practices. They are usually not a problem for conventional fluorescence microscopy because it does not have the resolution to reveal these artifacts. A sample might appear as perfect under a confocal microscopy and even under SIM, but higher resolution methods such as STED microscopy and STORM/PALM could reveal the clustering problem. Therefore, higher resolving power always demands more stringent sample preparation, very much resembling the case of electron microscopy. The three aforementioned issues apply to all superresolution microscopy techniques. For STORM/PALM in particular, the photophysics of the fluorophores can further exacerbate the clustering problem. Many organic dyes can undergo repetitive switching (Bates et al., 2007; Dempsey et al., 2011; Heilemann et al., 2008). With long acquisition time, multiple points are generated the same fluorophore (Figure 2C). Although most photoactivatable fluorescent proteins can nominally be activated only once, their blinking behavior also creates a cluster of localization points (Figure 2D), which might be misinterpreted as clustering of the target protein (Annibale et al., 2011a). Therefore, it is important to develop analysis algorithms that can correctly interpret these single-molecule clusters in STORM/PALM superresolution images (Annibale et al., 2011b). Making Sense of Superresolution Images Single-molecule events are by nature quantized and stochastic: there is no such thing as half of a molecule, and reactions at single-molecule level happen by probabilities. These characteristics serve as the basis of STORM/PALM and also bring in fundamental differences between STORM/PALM and conventional fluorescence microscopy, making it inappropriate to interpret an STORM/PALM image without statistical analysis. One consequence of imaging at single-molecule level is that noise appears the same as single-molecule signal. For example, the intrinsic noise in a fluorescence image has a low probability to be misidentified as a single-molecule activation event, especially when the background is high and the fluorophore is dim. The resulting false localization point scatters around the superresolution image and should not be confused with real ones (Figure 3). More complicated is the nonspecific binding of fluorescent probes to the sample. Unlike the homogeneous background Figure 3. Noise in STORM/PALM (A) Zoomed-in view of a boxed region in Figure 2B, left panel, showing false localization points (arrows). Particularly, misidentification of two nearby fluorophores activated at the same time creates the false localization points in the region surrounded by three microtubules. (B) Inset of Figure 1A before correction for crosstalk. The crosstalk can be seen as mixed green and red localization points. Arrows point to localization points either from nonspecific antibody binding or from false identification of background noise. often detected by conventional fluorescence microscopy, STORM/PALM detects nonspecific probe binding as scattered, individual probe molecules. Unlike the individually scattered false localization points coming from image noise, repetitive activation or blinking of these fluorophores further renders them into a cluster of localization points (Figure 2C). Another situation in which false localization points appear is the crosstalk in multicolor STORM/PALM. Generally, STORM/ PALM has two different approaches for multicolor imaging. One approach is to use fluorescent proteins or dyes with different emission wavelengths (Bossi et al., 2008). The crosstalk can be negligible in this case unless their emission wavelengths are extremely close (Gunewardene et al., 2011). The other approach is to use fluorescent probes (often a pair of organic of dyes) that have the same emission wavelength but can be activated by different wavelengths of light (Bates et al., 2007; Huang et al., 2008a). This approach avoids the necessity to align different emission channels, which can be challenging at nanometer scale, but it incurs more crosstalk due to spontaneous activation (Dani et al., 2010). The crosstalk in STORM/PALM appears as points in one color mixed into another color (Figure 3B), which could misinterpreted as colocalization. Unfortunately, the usual approach of crosstalk correction by subtracting pixel values cannot be applied here. Instead, crosstalk subtraction needs to be conducted for each localization point by analyzing the density of different colors of points surround it (Bates et al., 2007; Dani et al., 2010). Crosstalk subtraction is one of the examples that demonstrate the challenges in STORM/PALM image analysis. These images usually consist of molecule coordinates instead of pixels, which is incompatible with almost any existing image processing routines. A simple way to adapt a STORM/PALM image to those existing analysis packages is to bin it into a one-dimensional 1106 Developmental Cell 23, December 11, 2012 ª2012 Elsevier Inc.
5 histogram or a two-dimensional pixelated image based on the number of localization points within each bin or pixel. Calculation of subtraction, correlation, and shape fitting can then be performed, as illustrated by the determination of the molecular architecture of focal adhesion complexes and synapses (Dani et al., 2010; Kanchanawong et al., 2010). In both cases, fitting the histogram built from many molecule positions has resolved different domains on the same protein molecule. The binning method has also been used for counting the number of synapses in a brain region (Beaudoin et al., 2012). More recently, methods have been developed to calculate correlation function directly from molecule coordinates, which have enabled quantitative analysis of protein clustering and protein-protein colocalization (Sengupta et al., 2011). We expect that more algorithms and software packages will be developed in the near future to facilitate the interpretation of STORM/PALM images. The Opportunities The development of an organism consists of three interconnected elements: communication between cells, differentiation and determination of the cell fate, and cell migration and morphogenesis. Understanding the mechanisms behind the development of multicellular organisms requires knowledge covering scales from a molecule to a whole organism. Therefore, a wide variety of biological and physical methods have been demanded for the study of developmental biology. Superresolution microscopy, with its resolution lies at the scale between molecules and organelles, adds perfectly to this toolbox. It reveals how molecules work inside a cell, thus allowing us to link our structural understandings of biomolecules to their functions in the cellular and tissue context. Cell Communication During the organism development, a cell constantly exchanges signal with other cells and the outside environment in order to sense as well as establish its developmental context. These signal exchanges are most mediated by receptor molecules on the plasma membrane, especially those in the Notch, Hedgehog, Wnt, TGF(beta), and JAK/STAT pathways (Guruharsha et al., 2012; Krauss, 2008). Adhesion molecules including neuroliginneurexin, cadherins, and DSCAM also play critical roles in controlling cell recognition and cell morphogenesis (Zipursky and Sanes, 2010). Many of these pathways involve combinatorial use of a relatively small number of signaling molecules among diverse cell types. In addition, the receptors and adhesion molecules are regulated by oligomerization, clustering, anchoring to scaffolding proteins, and partitioning into membrane compartments. Understanding these regulation mechanisms calls for a method to uncover molecular interactions happening at the nanometer-to-micrometer scale. The planar geometry of the plasma membrane has made membrane receptors extensively studied targets by STORM/ PALM. These studies have been focused on the receptor clustering (Greenfield et al., 2009; Scarselli et al., 2012), participation of receptors in different lipid domains (Hess et al., 2007; Sengupta et al., 2011), and using single-particle tracking to monitor receptor diffusion (Hess et al., 2007; Manley et al., 2008). Many quantitative analysis methods have been developed for these studies. The next challenges would be to probe membrane receptors in the native tissue context. The 3D and multicolor imaging capability of STORM/PALM also makes it a powerful tool for studying the scaffolding and compartmentalizing of signaling molecules. For example, the composition and spatial distribution of neurotransmitter receptors in the postsynaptic density has been characterized by 3D STORM/PALM in the mouse main olfactory bulb and accessory olfactory bulb, revealing the different maturation states of synapses in these two brain regions (Dani et al., 2010). Similar strategies could be applied to other development-related signaling compartments, too, such as the machinery that controls the signaling molecule transportation and localization in the primary cilium. Cell Fate Determination One of the key processes during development is cell differentiation, which is achieved through the integration of external and internal signals by a complicated transcription, translation, and epigenetic regulation network. Compared to the success of microarray and sequencing techniques, imaging has been very much underexplored in studying cell fate determination. This situation can be partially attributed to the fact that much of the information that controls differentiation regulation lies in the DNA and RNA sequence, which perfectly fits microarray and sequencing analysis. Nevertheless, the capability of imaging techniques to probe dynamic processes in individual cells within a heterogeneous population is still uniquely advantageous. As has been mentioned earlier, STORM/PALM can routinely achieve 25 nm resolution, which corresponds to just about 70 base pairs for a linear stretch of double-strand DNA. Therefore, superresolution microscopy might ultimately enable us to map the DNA sequence space to the physical space in the nucleus. This capability could provide the opportunity to examine how gene expression is regulated by chromatin packaging and spatial organization, ranging from nucleosome scale (e.g., heterochromatin formation) to gene level (e.g., allelic exclusion; Yang and Kuroda, 2007) to whole chromosome scale (e.g., X-inactivation). Most of the chromatin STORM/PALM experiments so far rely on tagging histone proteins (Gunkel et al., 2009; Matsuda et al., 2010; Watanabe et al., 2011; Wombacher et al., 2010), although the development of small molecule probes for DNA and RNA labeling (Benke and Manley, 2012; Flors et al., 2009; Zessin et al., 2012) could further improve the effective spatial resolution. Another opportunity is to understand how nucleic acids interact with proteins in a cell. STORM/PALM has already demonstrated its potential in understanding how nucleoidassociated proteins help organizing Escherichia coli chromatin (Wang et al., 2011) and in revealing the interaction of centromere-associated histones (Ribeiro et al., 2010) and chromatin-remodeling proteins (Gunkel et al., 2009) with DNA. Sequence-specific labeling of DNA could be critical to these applications. In addition to fluorescence in situ hybridization (FISH), which has already been used in superresolution microscopy in a number of cases (Markaki et al., 2012; van de Corput et al., 2012; Weiland et al., 2011), newly developed labeling methods with minimal disruption to the structure of chromosomes will be greatly appreciated. Cell Morphogenesis and Migration Cells exhibit diverse morphologies, ranging from simple flats such as epithelial cells to extremely complicated shapes such Developmental Cell 23, December 11, 2012 ª2012 Elsevier Inc. 1107
6 as neurons. During development, these different cells sometimes migrate over a long distance to their final locations. Guided by both external signals and internal control networks, cell morphogenesis and migration involve protrusive structures such as filopodia and lamellipodia, cytoskeletons that undergo dynamic reorganization, and mechanical anchors such as focal adhesion and tight junctions. Many of these structures consist of highly organized protein complexes. Dissecting the architecture of these complexes presents a challenge for both conventional fluorescence microscopy (for the lack of spatial resolution) and electron microscopy (for the lack of protein specificity). Therefore, we need a technique that can fill in the gap between molecule-level knowledge generated by structural biology and biochemistry and cell-to-tissue level studies in cell and developmental biology. Superresolution microscopy comes in at the perfect position to bridge this gap. Cytoskeletal structures such as microtubules (Aquino et al., 2011; Bates et al., 2007; Heilemann et al., 2008; Huang et al., 2008b) and actin network (Frost et al., 2010; Izeddin et al., 2011; Xu et al., 2012) have long been the most popular imaging target for superresolution microscopy study. The morphology of the cell can also be defined with membrane stains (Shim et al., 2012) and membrane-anchored proteins (Aquino et al., 2011; Lakadamyali et al., 2012; Ries et al., 2012). STORM/PALM has revealed the multilayered molecular architecture of focal adhesions (Kanchanawong et al., 2010) and synaptic terminals (Dani et al., 2010), both demonstrating the capability to resolve different domains on the same protein molecule and, thus, the orientation of the protein in the complex. What can be even more helpful in the future is to combine superresolution structural imaging with STORM/PALM with functional imaging such as fluorescence resonance energy transfer (FRET). For example, combining superresolution microscopy and FRET probes reporting the activity of the Rho family of GTPases could uncover the mechanism of how they regulate cytoskeleton dynamics in a spatial-temporally defined manner (Kamiyama and Chiba, 2009). Conclusions Superresolution microscopy is still a young field under rapid development. Considering the immense number of questions awaiting answers in developmental biology, it is impossible for us to give an exhaustive list of what superresolution microscopy can do now and what new opportunities it will create in the future. As closer collaborations have started to form between research groups on the technology side and those on the biology side, and an ever-growing number of biology labs begins to gain access to superresolution microscopy through home-built instruments, imaging cores, and commercial products, we are confident that this new technology will lead to many new discoveries and new insights in the coming years. ACKNOWLEDGMENTS B.H. received the Searle Scholarship, the Packard Fellowship for Science Engineering, and the National Institutes of Health Director s New Innovator Award (1DP2OD ). REFERENCES Annibale, P., Vanni, S., Scarselli, M., Rothlisberger, U., and Radenovic, A. (2011a). Identification of clustering artifacts in photoactivated localization microscopy. Nat. Methods 8, Annibale, P., Vanni, S., Scarselli, M., Rothlisberger, U., and Radenovic, A. (2011b). Quantitative photo activated localization microscopy: unraveling the effects of photoblinking. PLoS ONE 6, e Aquino, D., Schönle, A., Geisler, C., Middendorff, C.V., Wurm, C.A., Okamura, Y., Lang, T., Hell, S.W., and Egner, A. (2011). Two-color nanoscopy of threedimensional volumes by 4Pi detection of stochastically switched fluorophores. Nat. Methods 8, Bates, M., Huang, B., Dempsey, G.T., and Zhuang, X. (2007). Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science 317, Beaudoin, G.M., 3rd, Schofield, C.M., Nuwal, T., Zang, K., Ullian, E.M., Huang, B., and Reichardt, L.F. (2012). Afadin, a Ras/Rap effector that controls cadherin function, promotes spine and excitatory synapse density in the hippocampus. J. Neurosci. 32, Benke, A., and Manley, S. (2012). Live-cell dstorm of cellular DNA based on direct DNA labeling. ChemBioChem 13, Betzig, E., Patterson, G.H., Sougrat, R., Lindwasser, O.W., Olenych, S., Bonifacino, J.S., Davidson, M.W., Lippincott-Schwartz, J., and Hess, H.F. (2006). Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, Bossi, M., Fölling, J., Belov, V.N., Boyarskiy, V.P., Medda, R., Egner, A., Eggeling, C., Schönle, A., and Hell, S.W. (2008). Multicolor far-field fluorescence nanoscopy through isolated detection of distinct molecular species. Nano Lett. 8, Burnette, D.T., Sengupta, P., Dai, Y.H., Lippincott-Schwartz, J., and Kachar, B. (2011). Bleaching/blinking assisted localization microscopy for superresolution imaging using standard fluorescent molecules. Proc. Natl. Acad. Sci. USA 108, Cella Zanacchi, F., Lavagnino, Z., Perrone Donnorso, M., Del Bue, A., Furia, L., Faretta, M., and Diaspro, A. (2011). Live-cell 3D super-resolution imaging in thick biological samples. Nat. Methods 8, Dani, A., Huang, B., Bergan, J., Dulac, C., and Zhuang, X. (2010). Superresolution imaging of chemical synapses in the brain. Neuron 68, Dedecker, P., Mo, G.C., Dertinger, T., and Zhang, J. (2012). Widely accessible method for superresolution fluorescence imaging of living systems. Proc. Natl. Acad. Sci. USA 109, Dempsey, G.T., Vaughan, J.C., Chen, K.H., Bates, M., and Zhuang, X. (2011). Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat. Methods 8, Denk, W., Strickler, J.H., and Webb, W.W. (1990). Two-photon laser scanning fluorescence microscopy. Science 248, Dertinger, T., Colyer, R., Iyer, G., Weiss, S., and Enderlein, J. (2009). Fast, background-free, 3D super-resolution optical fluctuation imaging (SOFI). Proc. Natl. Acad. Sci. USA 106, Egner, A., Geisler, C., von Middendorff, C., Bock, H., Wenzel, D., Medda, R., Andresen, M., Stiel, A.C., Jakobs, S., Eggeling, C., et al. (2007). Fluorescence nanoscopy in whole cells by asynchronous localization of photoswitching emitters. Biophys. J. 93, Fitzgerald, J.E., Lu, J., and Schnitzer, M.J. (2012). Estimation theoretic measure of resolution for stochastic localization microscopy. Phys. Rev. Lett. 109, Flors, C., Ravarani, C.N., and Dryden, D.T. (2009). Super-resolution imaging of DNA labelled with intercalating dyes. ChemPhysChem 10, Fölling, J., Bossi, M., Bock, H., Medda, R., Wurm, C.A., Hein, B., Jakobs, S., Eggeling, C., and Hell, S.W. (2008). Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat. Methods 5, Developmental Cell 23, December 11, 2012 ª2012 Elsevier Inc.
7 Frost, N.A., Shroff, H., Kong, H., Betzig, E., and Blanpied, T.A. (2010). Singlemolecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron 67, Girkin, J.M., Poland, S., and Wright, A.J. (2009). Adaptive optics for deeper imaging of biological samples. Curr. Opin. Biotechnol. 20, Greenfield, D., McEvoy, A.L., Shroff, H., Crooks, G.E., Wingreen, N.S., Betzig, E., and Liphardt, J. (2009). Self-organization of the Escherichia coli chemotaxis network imaged with super-resolution light microscopy. PLoS Biol. 7, e Gunewardene, M.S., Subach, F.V., Gould, T.J., Penoncello, G.P., Gudheti, M.V., Verkhusha, V.V., and Hess, S.T. (2011). Superresolution imaging of multiple fluorescent proteins with highly overlapping emission spectra in living cells. Biophys. J. 101, Gunkel, M., Erdel, F., Rippe, K., Lemmer, P., Kaufmann, R., Hörmann, C., Amberger, R., and Cremer, C. (2009). Dual color localization microscopy of cellular nanostructures. Biotechnol. J. 4, Guruharsha, K.G., Kankel, M.W., and Artavanis-Tsakonas, S. (2012). The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, Gustafsson, M.G.L. (2000). Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, Gustafsson, M.G.L. (2005). Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl. Acad. Sci. USA 102, Hama, H., Kurokawa, H., Kawano, H., Ando, R., Shimogori, T., Noda, H., Fukami, K., Sakaue-Sawano, A., and Miyawaki, A. (2011). Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 14, Heilemann, M., van de Linde, S., Schüttpelz, M., Kasper, R., Seefeldt, B., Mukherjee, A., Tinnefeld, P., and Sauer, M. (2008). Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. Engl. 47, Hell, S.W. (2007). Far-field optical nanoscopy. Science 316, Hell, S.W. (2009). Microscopy and its focal switch. Nat. Methods 6, Hell, S.W., and Wichmann, J. (1994). Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, Hess, S.T., Girirajan, T.P.K., and Mason, M.D. (2006). Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 91, Hess, S.T., Gould, T.J., Gudheti, M.V., Maas, S.A., Mills, K.D., and Zimmerberg, J. (2007). Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc. Natl. Acad. Sci. USA 104, Holden, S.J., Uphoff, S., and Kapanidis, A.N. (2011). DAOSTORM: an algorithm for high- density super-resolution microscopy. Nat. Methods 8, Huang, B., Jones, S.A., Brandenburg, B., and Zhuang, X. (2008a). Whole-cell 3D STORM reveals interactions between cellular structures with nanometerscale resolution. Nat. Methods 5, Huang, B., Wang, W., Bates, M., and Zhuang, X. (2008b). Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, Huang, B., Bates, M., and Zhuang, X. (2009). Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 78, Huang, B., Babcock, H., and Zhuang, X. (2010). Breaking the diffraction barrier: super-resolution imaging of cells. Cell 143, Huang, F., Schwartz, S.L., Byars, J.M., and Lidke, K.A. (2011). Simultaneous multiple-emitter fitting for single molecule super-resolution imaging. Biomed. Opt. Express 2, Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J., and Stelzer, E.H. (2004). Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, Izeddin, I., Specht, C.G., Lelek, M., Darzacq, X., Triller, A., Zimmer, C., and Dahan, M. (2011). Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS ONE 6, e Izeddin, I., El Beheiry, M., Andilla, J., Ciepielewski, D., Darzacq, X., and Dahan, M. (2012). PSF shaping using adaptive optics for three-dimensional singlemolecule super-resolution imaging and tracking. Opt. Express 20, Jones, S.A., Shim, S.H., He, J., and Zhuang, X. (2011). Fast, three-dimensional super-resolution imaging of live cells. Nat. Methods 8, Juette, M.F., Gould, T.J., Lessard, M.D., Mlodzianoski, M.J., Nagpure, B.S., Bennett, B.T., Hess, S.T., and Bewersdorf, J. (2008). Three-dimensional sub-100 nm resolution fluorescence microscopy of thick samples. Nat. Methods 5, Kamiyama, D., and Chiba, A. (2009). Endogenous activation patterns of Cdc42 GTPase within Drosophila embryos. Science 324, Kanchanawong, P., Shtengel, G., Pasapera, A.M., Ramko, E.B., Davidson, M.W., Hess, H.F., and Waterman, C.M. (2010). Nanoscale architecture of integrin-based cell adhesions. Nature 468, Klar, T.A., and Hell, S.W. (1999). Subdiffraction resolution in far-field fluorescence microscopy. Opt. Lett. 24, Klein, T., Löschberger, A., Proppert, S., Wolter, S., van de Linde, S.V., and Sauer, M. (2011). Live-cell dstorm with SNAP-tag fusion proteins. Nat. Methods 8, 7 9. Krauss, G. (2008). Biochemistry of Signal Transduction and Regulation, Fourth Edition (New York: Wiley-VHC). Lakadamyali, M., Babcock, H., Bates, M., Zhuang, X., and Lichtman, J. (2012). 3D multicolor super-resolution imaging offers improved accuracy in neuron tracing. PLoS ONE 7, e Lippincott-Schwartz, J., and Patterson, G.H. (2009). Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging. Trends Cell Biol. 19, Manley, S., Gillette, J.M., Patterson, G.H., Shroff, H., Hess, H.F., Betzig, E., and Lippincott-Schwartz, J. (2008). High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, Markaki, Y., Smeets, D., Fiedler, S., Schmid, V.J., Schermelleh, L., Cremer, T., and Cremer, M. (2012). The potential of 3D-FISH and super-resolution structured illumination microscopy for studies of 3D nuclear architecture: 3D structured illumination microscopy of defined chromosomal structures visualized by 3D (immuno)-fish opens new perspectives for studies of nuclear architecture. Bioessays 34, Matsuda, A., Shao, L., Boulanger, J., Kervrann, C., Carlton, P.M., Kner, P., Agard, D., and Sedat, J.W. (2010). Condensed mitotic chromosome structure at nanometer resolution using PALM and EGFP- histones. PLoS ONE 5, e Micheva, K.D., and Smith, S.J. (2007). Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron 55, Mukamel, E.A., Babcock, H., and Zhuang, X. (2012). Statistical deconvolution for superresolution fluorescence microscopy. Biophys. J. 102, Nanguneri, S., Flottmann, B., Horstmann, H., Heilemann, M., and Kuner, T. (2012). Three-dimensional, tomographic super-resolution fluorescence imaging of serially sectioned thick samples. PLoS ONE 7, e Quan, T.W., Zhu, H.Y., Liu, X.M., Liu, Y.F., Ding, J.P., Zeng, S.Q., and Huang, Z.L. (2011). High-density localization of active molecules using Structured Sparse Model and Bayesian Information Criterion. Opt. Express 19, Ribeiro, S.A., Vagnarelli, P., Dong, Y., Hori, T., McEwen, B.F., Fukagawa, T., Flors, C., and Earnshaw, W.C. (2010). A super-resolution map of the vertebrate kinetochore. Proc. Natl. Acad. Sci. USA 107, Developmental Cell 23, December 11, 2012 ª2012 Elsevier Inc. 1109
8 Ries, J., Kaplan, C., Platonova, E., Eghlidi, H., and Ewers, H. (2012). A simple, versatile method for GFP-based super-resolution microscopy via nanobodies. Nat. Methods 9, Rust, M.J., Bates, M., and Zhuang, X. (2006). Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, Scarselli, M., Annibale, P., and Radenovic, A. (2012). Cell type-specific b2-adrenergic receptor clusters identified using photoactivated localization microscopy are not lipid raft related, but depend on actin cytoskeleton integrity. J. Biol. Chem. 287, Sengupta, P., Jovanovic-Talisman, T., Skoko, D., Renz, M., Veatch, S.L., and Lippincott-Schwartz, J. (2011). Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat. Methods 8, Sharonov, A., and Hochstrasser, R.M. (2006). Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl. Acad. Sci. USA 103, Shim, S.H., Xia, C., Zhong, G., Babcock, H.P., Vaughan, J.C., Huang, B., Wang, X., Xu, C., Bi, G.Q., and Zhuang, X. (2012). Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc. Natl. Acad. Sci. USA 109, Shroff, H., Galbraith, C.G., Galbraith, J.A., White, H., Gillette, J., Olenych, S., Davidson, M.W., and Betzig, E. (2007). Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc. Natl. Acad. Sci. USA 104, Shroff, H., Galbraith, C.G., Galbraith, J.A., and Betzig, E. (2008). Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat. Methods 5, Shtengel, G., Galbraith, J.A., Galbraith, C.G., Lippincott-Schwartz, J., Gillette, J.M., Manley, S., Sougrat, R., Waterman, C.M., Kanchanawong, P., Davidson, M.W., et al. (2009). Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc. Natl. Acad. Sci. USA 106, Thompson, R.E., Larson, D.R., and Webb, W.W. (2002). Precise nanometer localization analysis for individual fluorescent probes. Biophys. J. 82, Tokunaga, M., Imamoto, N., and Sakata-Sogawa, K. (2008). Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods 5, van de Corput, M.P., de Boer, E., Knoch, T.A., van Cappellen, W.A., Quintanilla, A., Ferrand, L., and Grosveld, F.G. (2012). Super-resolution imaging reveals 3D folding dynamics of the b-globin locus upon gene activation. J. Cell Sci.. Vaziri, A., Tang, J.Y., Shroff, H., and Shank, C.V. (2008). Multilayer threedimensional super resolution imaging of thick biological samples. Proc. Natl. Acad. Sci. USA 105, Wang, W., Li, G.W., Chen, C., Xie, X.S., and Zhuang, X. (2011). Chromosome organization by a nucleoid-associated protein in live bacteria. Science 333, Watanabe, S., Punge, A., Hollopeter, G., Willig, K.I., Hobson, R.J., Davis, M.W., Hell, S.W., and Jorgensen, E.M. (2011). Protein localization in electron micrographs using fluorescence nanoscopy. Nat. Methods 8, Weiland, Y., Lemmer, P., and Cremer, C. (2011). Combining FISH with localisation microscopy: Super-resolution imaging of nuclear genome nanostructures. Chromosome Res. 19, Wombacher, R., Heidbreder, M., van de Linde, S., Sheetz, M.P., Heilemann, M., Cornish, V.W., and Sauer, M. (2010). Live-cell super-resolution imaging with trimethoprim conjugates. Nat. Methods 7, Wu, M., Huang, B., Graham, M., Raimondi, A., Heuser, J.E., Zhuang, X.W., and De Camilli, P. (2010). Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nat. Cell Biol. 12, Xu, K., Babcock, H.P., and Zhuang, X. (2012). Dual-objective STORM reveals three-dimensional filament organization in the actin cytoskeleton. Nat. Methods 9, Yang, P.K., and Kuroda, M.I. (2007). Noncoding RNAs and intranuclear positioning in monoallelic gene expression. Cell 128, York, A.G., Ghitani, A., Vaziri, A., Davidson, M.W., and Shroff, H. (2011). Confined activation and subdiffractive localization enables whole-cell PALM with genetically expressed probes. Nat. Methods 8, Zessin, P.J., Finan, K., and Heilemann, M. (2012). Super-resolution fluorescence imaging of chromosomal DNA. J. Struct. Biol. 177, Zhu, L., Zhang, W., Elnatan, D., and Huang, B. (2012). Faster STORM using compressed sensing. Nat. Methods 9, Zipursky, S.L., and Sanes, J.R. (2010). Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell 143, Developmental Cell 23, December 11, 2012 ª2012 Elsevier Inc.
STORM/PALM. Super Resolution Microscopy 10/31/2011. Looking into microscopic world of life
 Super Resolution Microscopy STORM/PALM Bo Huang Department of Pharmaceutical Chemistry, UCSF CSHL Quantitative Microscopy, 1/31/211 Looking into microscopic world of life 1 µm 1 µm 1 nm 1 nm 1 nm 1 Å Naked
Super Resolution Microscopy STORM/PALM Bo Huang Department of Pharmaceutical Chemistry, UCSF CSHL Quantitative Microscopy, 1/31/211 Looking into microscopic world of life 1 µm 1 µm 1 nm 1 nm 1 nm 1 Å Naked
SIM SSIM. nanoscopy RESOLFT. Super-resolution STORM GSDIM. dstorm PALMIRA FPALM PALM PAINT SPRAIPAINT SOFI BALM CALM. Bo Huang
 STEDGSD STORM SOFI nanoscopy GSDIM PALMIRA SMACM BBB PAINT SPRAIPAINT CALM RESOLFT BALM SIM SSIM Super-resolution Bo Huang 2013.08.01 dstorm FPALM PALM 50 years to extend the resolution Confocal microscopy
STEDGSD STORM SOFI nanoscopy GSDIM PALMIRA SMACM BBB PAINT SPRAIPAINT CALM RESOLFT BALM SIM SSIM Super-resolution Bo Huang 2013.08.01 dstorm FPALM PALM 50 years to extend the resolution Confocal microscopy
Q&A: Single-molecule localization microscopy for biological imaging
 Q U E S T I O N & ANSWER Q&A: Single-molecule localization microscopy for biological imaging Ann L McEvoy 1, Derek Greenfield 1,2,5, Mark Bates 3 and Jan Liphardt 1,2,4 * Open Access Why is it important
Q U E S T I O N & ANSWER Q&A: Single-molecule localization microscopy for biological imaging Ann L McEvoy 1, Derek Greenfield 1,2,5, Mark Bates 3 and Jan Liphardt 1,2,4 * Open Access Why is it important
Introduction CHAPTER 1
 CHAPTER 1 Introduction 1.1 Light Microscopy The light microscope is one of the significant inventions in the history of humankind that, along with the telescope, played a central role in the Scientific
CHAPTER 1 Introduction 1.1 Light Microscopy The light microscope is one of the significant inventions in the history of humankind that, along with the telescope, played a central role in the Scientific
Super-resolution microscopy at a glance
 ARTICLE SERIES: Imaging Cell Science at a Glance 1607 Super-resolution microscopy at a glance Catherine G. Galbraith 1, * and James A. Galbraith 2, * 1 National Institute of Health, 1 NICHD and 2 NINDS,
ARTICLE SERIES: Imaging Cell Science at a Glance 1607 Super-resolution microscopy at a glance Catherine G. Galbraith 1, * and James A. Galbraith 2, * 1 National Institute of Health, 1 NICHD and 2 NINDS,
Fluorescence Nanoscopy
 Fluorescence Nanoscopy Keith A. Lidke University of New Mexico panda3.phys.unm.edu/~klidke/index.html Optical Microscopy http://en.wikipedia.org/wiki/k%c3%b6hler_illumination 30 µm Fluorescent Probes Michalet
Fluorescence Nanoscopy Keith A. Lidke University of New Mexico panda3.phys.unm.edu/~klidke/index.html Optical Microscopy http://en.wikipedia.org/wiki/k%c3%b6hler_illumination 30 µm Fluorescent Probes Michalet
Bi177 - Lecture 13 Microscopy Outside the Box. Fluorescence Nanoscopy TIRF 4-pi STED STORM/PALM
 Bi177 - Lecture 13 Microscopy Outside the Box Fluorescence Nanoscopy TIRF 4-pi STED STORM/PALM The diffraction limit: Abbe s law The Problem Diffraction limit 100x larger than molecular scale! Green Fluorescent
Bi177 - Lecture 13 Microscopy Outside the Box Fluorescence Nanoscopy TIRF 4-pi STED STORM/PALM The diffraction limit: Abbe s law The Problem Diffraction limit 100x larger than molecular scale! Green Fluorescent
PALM/STORM, BALM, STED
 PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
Tracking sub-microscopic protein organisation at the plasma membrane of live cells using triple-colour superresolution
 Tracking sub-microscopic protein organisation at the plasma membrane of live cells using triple-colour superresolution microscopy Jacob Piehler Division of Biophysics, University of Osnabrück, Germany
Tracking sub-microscopic protein organisation at the plasma membrane of live cells using triple-colour superresolution microscopy Jacob Piehler Division of Biophysics, University of Osnabrück, Germany
Localization-Based Super-Resolution Light Microscopy
 Kristin A. Gabor, 1,2,3 Mudalige S. Gunewardene, 1 David Santucci, 4 and Samuel T. Hess 1,3, * 1 Department of Physics and Astronomy, 120 Bennett Hall, University of Maine, Orono, ME 04469 2 Department
Kristin A. Gabor, 1,2,3 Mudalige S. Gunewardene, 1 David Santucci, 4 and Samuel T. Hess 1,3, * 1 Department of Physics and Astronomy, 120 Bennett Hall, University of Maine, Orono, ME 04469 2 Department
Introduction to N-STORM
 Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Enlightening G-protein-coupled receptors on the plasma membrane using super-resolution photoactivated localization microscopy
 G-Protein-Coupled Receptors: from Structural Insights to Functional Mechanisms 191 Enlightening G-protein-coupled receptors on the plasma membrane using super-resolution photoactivated localization microscopy
G-Protein-Coupled Receptors: from Structural Insights to Functional Mechanisms 191 Enlightening G-protein-coupled receptors on the plasma membrane using super-resolution photoactivated localization microscopy
Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc.
 15/05/2012 Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc. Prof. Dr. Rainer Duden duden@bio.uni-luebeck.de 1 Using conventional light microscopy resolution is limited
15/05/2012 Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc. Prof. Dr. Rainer Duden duden@bio.uni-luebeck.de 1 Using conventional light microscopy resolution is limited
A Brief History of Light Microscopy And How It Transformed Biomedical Research
 A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
Fast, three-dimensional super-resolution imaging of live cells
 Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Super Resolution Imaging Solution Provider. Imaging Future
 Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
Measure of surface protein mobility with u-paint technique
 Measure of surface protein mobility with u-paint technique How dynamic image can solve the situation? Random distribution or cluster? Why live super-resolution microscopy can solve the situation With mobility
Measure of surface protein mobility with u-paint technique How dynamic image can solve the situation? Random distribution or cluster? Why live super-resolution microscopy can solve the situation With mobility
Nanoscale measurement examples in biology: Sub-diffractive imaging & the fly brain challenge
 Nanoscale measurement examples in biology: Sub-diffractive imaging & the fly brain challenge Harald Hess, HHMI Janelia Farm Introduction to HHMI Janelia Farms Sample: Nerves, Worm Brains, Fly Brains, Rodent
Nanoscale measurement examples in biology: Sub-diffractive imaging & the fly brain challenge Harald Hess, HHMI Janelia Farm Introduction to HHMI Janelia Farms Sample: Nerves, Worm Brains, Fly Brains, Rodent
Journal of Biotechnology
 Journal of Biotechnology 149 (2010) 243 251 Contents lists available at ScienceDirect Journal of Biotechnology journal homepage: www.elsevier.com/locate/jbiotec Fluorescence microscopy beyond the diffraction
Journal of Biotechnology 149 (2010) 243 251 Contents lists available at ScienceDirect Journal of Biotechnology journal homepage: www.elsevier.com/locate/jbiotec Fluorescence microscopy beyond the diffraction
Confocal Microscopy & Imaging Technology. Yan Wu
 Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Localization Microscopy
 Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Biophysical Journal Volume 107 October Super-resolution Microscopy Approaches for Live Cell Imaging
 Biophysical Journal Volume 107 October 2014 1777 1784 1777 Biophysical Review Super-resolution Microscopy Approaches for Live Cell Imaging Antoine G. Godin, 1,2 Brahim Lounis, 1,2 and Laurent Cognet 1,2,
Biophysical Journal Volume 107 October 2014 1777 1784 1777 Biophysical Review Super-resolution Microscopy Approaches for Live Cell Imaging Antoine G. Godin, 1,2 Brahim Lounis, 1,2 and Laurent Cognet 1,2,
Accelerated super-resolution imaging with FRET-PAINT
 Lee et al. Molecular Brain (2017) 10:63 DOI 10.1186/s13041-017-0344-5 RESEARCH Accelerated super-resolution imaging with FRET-PAINT Jongjin Lee 1,2, Sangjun Park 1,2, Wooyoung Kang 1,2 and Sungchul Hohng
Lee et al. Molecular Brain (2017) 10:63 DOI 10.1186/s13041-017-0344-5 RESEARCH Accelerated super-resolution imaging with FRET-PAINT Jongjin Lee 1,2, Sangjun Park 1,2, Wooyoung Kang 1,2 and Sungchul Hohng
Super-Resolution Localization Microscopy
 APPLICATION NOTE Super-Resolution Localization Microscopy Light microscopy techniques have been vital to our understanding of biological structures and systems since their invention in the late 16 th Century.
APPLICATION NOTE Super-Resolution Localization Microscopy Light microscopy techniques have been vital to our understanding of biological structures and systems since their invention in the late 16 th Century.
Multiplexed imaging using same species primary antibodies with signal amplification
 Multiplexed imaging using same species primary antibodies with signal amplification Yu Wang 1,2, Wenxin Xie 1,2, Richie E. Kohman 1 and George M. Church 1,2 1. Wyss Institute for Biologically Inspired
Multiplexed imaging using same species primary antibodies with signal amplification Yu Wang 1,2, Wenxin Xie 1,2, Richie E. Kohman 1 and George M. Church 1,2 1. Wyss Institute for Biologically Inspired
Visualizing Cells Molecular Biology of the Cell - Chapter 9
 Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
SUPER-RESOLUTION MICROSCOPY. Dr. Nathalie Garin
 SUPER-RESOLUTION MICROSCOPY Dr. Nathalie Garin Content Motivation for superresolution Superresolution, nanoscopy, : definition Structured Illumination Microscopy (SIM) Localization microscopy STimulated
SUPER-RESOLUTION MICROSCOPY Dr. Nathalie Garin Content Motivation for superresolution Superresolution, nanoscopy, : definition Structured Illumination Microscopy (SIM) Localization microscopy STimulated
Introduction to Computational Fluorescence Microscopy!
 Introduction to Computational Fluorescence Microscopy! EE367/CS448I: Computational Imaging and Display! stanford.edu/class/ee367! Lecture 13! Gordon Wetzstein! Stanford University! Midterm! Tuesday, Feb
Introduction to Computational Fluorescence Microscopy! EE367/CS448I: Computational Imaging and Display! stanford.edu/class/ee367! Lecture 13! Gordon Wetzstein! Stanford University! Midterm! Tuesday, Feb
a) JOURNAL OF BIOLOGICAL CHEMISTRY b) PNAS c) NATURE
 a) JOURNAL OF BIOLOGICAL CHEMISTRY b) c) d) ........................ JOURNAL OF BIOLOGICAL CHEMISTRY MOLECULAR PHARMACOLOGY TRENDS IN PHARMACOLOGICAL S AMERICAN JOURNAL OF PHYSIOLOGY-HEART AND CIRCULATORY
a) JOURNAL OF BIOLOGICAL CHEMISTRY b) c) d) ........................ JOURNAL OF BIOLOGICAL CHEMISTRY MOLECULAR PHARMACOLOGY TRENDS IN PHARMACOLOGICAL S AMERICAN JOURNAL OF PHYSIOLOGY-HEART AND CIRCULATORY
Breaking the Diffraction Barrier: Super-Resolution Imaging of Cells
 Leading Edge Primer Breaking the Diffraction Barrier: Super-Resolution Imaging of Cells Bo Huang, 1 Hazen Babcock, 2 and Xiaowei Zhuang 2,3, * 1 Department of Pharmaceutical Chemistry and Department of
Leading Edge Primer Breaking the Diffraction Barrier: Super-Resolution Imaging of Cells Bo Huang, 1 Hazen Babcock, 2 and Xiaowei Zhuang 2,3, * 1 Department of Pharmaceutical Chemistry and Department of
Supplementary Information
 Supplementary Information STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA Iddo Heller, Gerrit Sitters, Onno D. Broekmans, Géraldine Farge, Carolin Menges,
Supplementary Information STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA Iddo Heller, Gerrit Sitters, Onno D. Broekmans, Géraldine Farge, Carolin Menges,
SUPER-RESOLUTION MICROSCOPY
 University of Groningen Faculty of Mathematics and Natural Sciences Zernike Institute for Advanced Materials Nanoscience Research Paper Mykhailo Gerasymenko Top Master in Nanosience m.gerasymenko@student.rug.nl
University of Groningen Faculty of Mathematics and Natural Sciences Zernike Institute for Advanced Materials Nanoscience Research Paper Mykhailo Gerasymenko Top Master in Nanosience m.gerasymenko@student.rug.nl
Sample region with fluorescent labeled molecules
 FLUORESCENCE IMAGING I. Fluorescence-imaging with diffraction limited spots The resolution in optical microscopy has been hampered by the smallest spot possible (~ λ/2) that can be achieved by conventional
FLUORESCENCE IMAGING I. Fluorescence-imaging with diffraction limited spots The resolution in optical microscopy has been hampered by the smallest spot possible (~ λ/2) that can be achieved by conventional
Single-Particle Tracking Photoactivated Localization Microscopy for Mapping Single-Molecule Dynamics
 CHAPTER FIVE Single-Particle Tracking Photoactivated Localization Microscopy for Mapping Single-Molecule Dynamics Suliana Manley,* Jennifer M. Gillette, and Jennifer Lippincott-Schwartz Contents 1. Introduction
CHAPTER FIVE Single-Particle Tracking Photoactivated Localization Microscopy for Mapping Single-Molecule Dynamics Suliana Manley,* Jennifer M. Gillette, and Jennifer Lippincott-Schwartz Contents 1. Introduction
Abstract. Introduction
 Abstract The investigation of interactions between engineered nanostructures and biological systems is a key component in the assessment of potential environmental and health implications due to the increasing
Abstract The investigation of interactions between engineered nanostructures and biological systems is a key component in the assessment of potential environmental and health implications due to the increasing
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Post-expansion antibody delivery, after epitope-preserving homogenization.
 Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
8:00 pm David A. Agard, HHMI/University of California, San Francisco Welcome and introduction to the meeting
 Sunday, May 20 th 3:00 pm Check-in 6:00 pm Reception 7:00 pm Dinner 8:00 pm Session 1: Introduction 8:00 pm David A. Agard, HHMI/University of California, San Francisco Welcome and introduction to the
Sunday, May 20 th 3:00 pm Check-in 6:00 pm Reception 7:00 pm Dinner 8:00 pm Session 1: Introduction 8:00 pm David A. Agard, HHMI/University of California, San Francisco Welcome and introduction to the
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
ChIP-seq and RNA-seq. Farhat Habib
 ChIP-seq and RNA-seq Farhat Habib fhabib@iiserpune.ac.in Biological Goals Learn how genomes encode the diverse patterns of gene expression that define each cell type and state. Protein-DNA interactions
ChIP-seq and RNA-seq Farhat Habib fhabib@iiserpune.ac.in Biological Goals Learn how genomes encode the diverse patterns of gene expression that define each cell type and state. Protein-DNA interactions
Two-Photon Microscopy for Deep Tissue Imaging of Living Specimens
 for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
Rice/TCU REU on Computational Neuroscience. Fundamentals of Molecular Imaging
 Rice/TCU REU on Computational Neuroscience Fundamentals of Molecular Imaging June 2, 2009 Neal Waxham 713-500-5621 m.n.waxham@uth.tmc.edu Objectives Introduction to resolution in light microscopy Brief
Rice/TCU REU on Computational Neuroscience Fundamentals of Molecular Imaging June 2, 2009 Neal Waxham 713-500-5621 m.n.waxham@uth.tmc.edu Objectives Introduction to resolution in light microscopy Brief
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Super-resolution Microscopy
 Semr oc kwhi t epaperser i es : 1. Introduction Super-resolution Microscopy Fluorescence microscopy has revolutionized the study of biological samples. Ever since the invention of fluorescence microscopy
Semr oc kwhi t epaperser i es : 1. Introduction Super-resolution Microscopy Fluorescence microscopy has revolutionized the study of biological samples. Ever since the invention of fluorescence microscopy
Fluorescence microscopy
 Fluorescence microscopy 1 Fluorescence microscopies basic fluorescence, fluorophores Deconvolution Confocal Two-photon/multi-photon 4Pi Light sheet Total internal reflection STED FRAP/FLIP/FCS FRET PALM/STORM/iPALM
Fluorescence microscopy 1 Fluorescence microscopies basic fluorescence, fluorophores Deconvolution Confocal Two-photon/multi-photon 4Pi Light sheet Total internal reflection STED FRAP/FLIP/FCS FRET PALM/STORM/iPALM
Lab 5: Optical trapping and single molecule fluorescence
 Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope
 High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
Atto (8) UV-Violet (405 nm) 10mM MEA/OS (8) 8, 9 Highest performance 488nm-excitable dye for STORM
 Photons/ Synthetic Dyes 488 nm Vybrant Dye Cycle Violet 369 (B), ~488 (G) 437 (B), ~510 (G) 2409 (1) Glycerol/OS (1) 1-3 DAPI 364 (B), ~488 (G) 454 (B), ~510 (G) -- Glycerol/OS (4) 4 Hoescht 33342 350
Photons/ Synthetic Dyes 488 nm Vybrant Dye Cycle Violet 369 (B), ~488 (G) 437 (B), ~510 (G) 2409 (1) Glycerol/OS (1) 1-3 DAPI 364 (B), ~488 (G) 454 (B), ~510 (G) -- Glycerol/OS (4) 4 Hoescht 33342 350
7/14/2015. Single-cell-level measurements of transcription heterogeneity of highly mobile identical genes. Enrico Gratton and Paolo Annibale
 7//5 Single-cell-level measurements of transcription heterogeneity of highly mobile identical genes Enrico Gratton and Paolo Annibale Laboratory for Florescence Dynamics University of California, Irvine
7//5 Single-cell-level measurements of transcription heterogeneity of highly mobile identical genes Enrico Gratton and Paolo Annibale Laboratory for Florescence Dynamics University of California, Irvine
Nanoscale Spatial Organization of Prokaryotic Cells Studied by Super-Resolution Optical Microscopy. Andrea Lynn McEvoy
 Nanoscale Spatial Organization of Prokaryotic Cells Studied by Super-Resolution Optical Microscopy by Andrea Lynn McEvoy A dissertation submitted in partial satisfaction of the requirements for the degree
Nanoscale Spatial Organization of Prokaryotic Cells Studied by Super-Resolution Optical Microscopy by Andrea Lynn McEvoy A dissertation submitted in partial satisfaction of the requirements for the degree
Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng
 Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Super-Resolution Imaging System. Professionals serve professionals. Three-Dimensional Simultaneous Multi-Channel Super Resolution Imaging
 Professionals serve professionals Super-Resolution Imaging System Three-Dimensional Simultaneous Multi-Channel Super Resolution Imaging NanoBioImaging Ltd. Powerful Tool of Scientists Super-resolution
Professionals serve professionals Super-Resolution Imaging System Three-Dimensional Simultaneous Multi-Channel Super Resolution Imaging NanoBioImaging Ltd. Powerful Tool of Scientists Super-resolution
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity
 Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
PALM Microscope User Manual
 PALM Microscope User Manual Stephan Uphoff, Mathew Stracy, Federico Garza de Leon Microscope components: The laser excitation is delivered from a Toptica Multi Laser Engine with 405 nm, 488 nm, 561 nm,
PALM Microscope User Manual Stephan Uphoff, Mathew Stracy, Federico Garza de Leon Microscope components: The laser excitation is delivered from a Toptica Multi Laser Engine with 405 nm, 488 nm, 561 nm,
Stochastic Optical Reconstruction Microscopy (STORM): A Method for Superresolution Fluorescence Imaging
 Topic Introduction Stochastic Optical Reconstruction Microscopy (STORM): A Method for Superresolution Fluorescence Imaging Mark Bates, Sara A. Jones, and Xiaowei Zhuang The relatively low spatial resolution
Topic Introduction Stochastic Optical Reconstruction Microscopy (STORM): A Method for Superresolution Fluorescence Imaging Mark Bates, Sara A. Jones, and Xiaowei Zhuang The relatively low spatial resolution
Super-resolution Microscopy Approaches for Live Cell Imaging
 Super-resolution Microscopy Approaches for Live Cell Imaging Antoine Godin, Brahim Lounis, Laurent Cognet To cite this version: Antoine Godin, Brahim Lounis, Laurent Cognet. Super-resolution Microscopy
Super-resolution Microscopy Approaches for Live Cell Imaging Antoine Godin, Brahim Lounis, Laurent Cognet To cite this version: Antoine Godin, Brahim Lounis, Laurent Cognet. Super-resolution Microscopy
Transcription factor dynamics in the nucleus
 Transcription factor dynamics in the nucleus March 21, 2018 Luca Giorgetti txnlecture2018@fmi.ch Transcription factors bind to enhancer and promoter regions Adapted from Streubel & Bracken EMBO J 2015
Transcription factor dynamics in the nucleus March 21, 2018 Luca Giorgetti txnlecture2018@fmi.ch Transcription factors bind to enhancer and promoter regions Adapted from Streubel & Bracken EMBO J 2015
COPYRIGHTED MATERIAL. Tissue Preparation and Microscopy. General Concepts. Chemical Fixation CHAPTER 1
 CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
Vertico-SMI /SPDMphymod: Next Level of Super-Resolution Fluorescence Microscopy
 Work in your familiar GFP/YFP/RFP system from the first experiment to the nanoimage Bwcon business award winner: Inventor Prof Christoph Cremer Vertico-SMI /SPDMphymod: Next Level of Super-Resolution Fluorescence
Work in your familiar GFP/YFP/RFP system from the first experiment to the nanoimage Bwcon business award winner: Inventor Prof Christoph Cremer Vertico-SMI /SPDMphymod: Next Level of Super-Resolution Fluorescence
Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers
 Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
ChIP-seq and RNA-seq
 ChIP-seq and RNA-seq Biological Goals Learn how genomes encode the diverse patterns of gene expression that define each cell type and state. Protein-DNA interactions (ChIPchromatin immunoprecipitation)
ChIP-seq and RNA-seq Biological Goals Learn how genomes encode the diverse patterns of gene expression that define each cell type and state. Protein-DNA interactions (ChIPchromatin immunoprecipitation)
Multiphoton Microscopy: Seeing deeper and clearer
 Multiphoton Microscopy: Seeing deeper and clearer Since the invention of simple microscope by Leuwenhoek and Hooke in the 17th century, different types of light microscopy techniques (such as phase contrast,
Multiphoton Microscopy: Seeing deeper and clearer Since the invention of simple microscope by Leuwenhoek and Hooke in the 17th century, different types of light microscopy techniques (such as phase contrast,
F* techniques: FRAP, FLIP, FRET, FLIM,
 F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
Photoactivatable mcherry for high-resolution two-color fluorescence microscopy
 Photoactivatable mcherry for high-resolution two-color fluorescence microscopy Fedor V Subach 1 3, George H Patterson,3, Suliana Manley, Jennifer M Gillette, Jennifer Lippincott-Schwartz & Vladislav V
Photoactivatable mcherry for high-resolution two-color fluorescence microscopy Fedor V Subach 1 3, George H Patterson,3, Suliana Manley, Jennifer M Gillette, Jennifer Lippincott-Schwartz & Vladislav V
Microscopy. CS/CME/BioE/Biophys/BMI 279 Nov. 2, 2017 Ron Dror
 Microscopy CS/CME/BioE/Biophys/BMI 279 Nov. 2, 2017 Ron Dror 1 Outline Microscopy: the basics Fluorescence microscopy Resolution limits The diffraction limit Beating the diffraction limit 2 Microscopy:
Microscopy CS/CME/BioE/Biophys/BMI 279 Nov. 2, 2017 Ron Dror 1 Outline Microscopy: the basics Fluorescence microscopy Resolution limits The diffraction limit Beating the diffraction limit 2 Microscopy:
Measuring gene expression and protein production
 Measuring gene expression and protein production Module 3, Lecture 5! 20.109 Spring 2013! Lessons from Lecture 4 Always look for the recovery file! The myth of multitasking! task-switching taps processing
Measuring gene expression and protein production Module 3, Lecture 5! 20.109 Spring 2013! Lessons from Lecture 4 Always look for the recovery file! The myth of multitasking! task-switching taps processing
Fluorescence Nanoscopy 高甫仁 ) Institute of Biophotonics, National Yang Ming University. Outline
 Fluorescence Nanoscopy 高甫仁 ) Fu-Jen Kao ( 高甫仁 Institute of Biophotonics, National Yang Ming University Outline The Abbe s (diffraction) limit and nanoscopy Fundamentals and opportunities of FLIM/FRET Visualizing
Fluorescence Nanoscopy 高甫仁 ) Fu-Jen Kao ( 高甫仁 Institute of Biophotonics, National Yang Ming University Outline The Abbe s (diffraction) limit and nanoscopy Fundamentals and opportunities of FLIM/FRET Visualizing
T he recent widespread uptake of new super resolution techniques has revolutionized and invigorated molecular
 OPEN SUBJECT AREAS: SUPER-RESOLUTION MICROSCOPY SINGLE-MOLECULE BIOPHYSICS Received 16 October 2014 Accepted 11 December 2014 Published 21 January 2015 Correspondence and requests for materials should
OPEN SUBJECT AREAS: SUPER-RESOLUTION MICROSCOPY SINGLE-MOLECULE BIOPHYSICS Received 16 October 2014 Accepted 11 December 2014 Published 21 January 2015 Correspondence and requests for materials should
FRAUNHOFER IME SCREENINGPORT
 FRAUNHOFER IME SCREENINGPORT Detection technologies used in drug discovery Introduction Detection technologies in drug discovery is unlimited only a biased snapshot can be presented Differences can presented
FRAUNHOFER IME SCREENINGPORT Detection technologies used in drug discovery Introduction Detection technologies in drug discovery is unlimited only a biased snapshot can be presented Differences can presented
cell and tissue imaging by fluorescence microscopy
 cell and tissue imaging by fluorescence microscopy Steven NEDELLEC Plateforme Micropicell SFR Santé François Bonamy Nantes 1 A matter of size Limit of resolution 0.15mm aims: building the image of an object
cell and tissue imaging by fluorescence microscopy Steven NEDELLEC Plateforme Micropicell SFR Santé François Bonamy Nantes 1 A matter of size Limit of resolution 0.15mm aims: building the image of an object
Resolution of Microscopes Visible light is nm Dry lens(0.5na), green(530nm light)=0.65µm=650nm for oil lens (1.4NA) UV light (300nm) = 0.13µm f
 Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
Comparative Genomic Hybridization
 Comparative Genomic Hybridization Srikesh G. Arunajadai Division of Biostatistics University of California Berkeley PH 296 Presentation Fall 2002 December 9 th 2002 OUTLINE CGH Introduction Methodology,
Comparative Genomic Hybridization Srikesh G. Arunajadai Division of Biostatistics University of California Berkeley PH 296 Presentation Fall 2002 December 9 th 2002 OUTLINE CGH Introduction Methodology,
Quantitative Photo Activated Localization Microscopy: Unraveling the Effects of Photoblinking
 Quantitative Photo Activated Localization Microscopy: Unraveling the Effects of Photoblinking Paolo Annibale 1., Stefano Vanni 2., Marco Scarselli 1., Ursula Rothlisberger 2, Aleksandra Radenovic 1 * 1
Quantitative Photo Activated Localization Microscopy: Unraveling the Effects of Photoblinking Paolo Annibale 1., Stefano Vanni 2., Marco Scarselli 1., Ursula Rothlisberger 2, Aleksandra Radenovic 1 * 1
Special Techniques 1. Mark Scott FILM Facility
 Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Nature Methods: doi: /nmeth Supplementary Figure 1. Retention of RNA with LabelX.
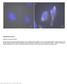 Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
Size Estimation of Protein Clusters in the Nanometer Range by Using Spatially Modulated Illumination Microscopy
 FORMATEX 2007 A. Méndez-Vilas and J. Díaz (Eds.) Size Estimation of Protein Clusters in the Nanometer Range by Using Spatially Modulated Illumination Microscopy U. J. Birk 1,2, I. Upmann 1, D. Toomre 3,
FORMATEX 2007 A. Méndez-Vilas and J. Díaz (Eds.) Size Estimation of Protein Clusters in the Nanometer Range by Using Spatially Modulated Illumination Microscopy U. J. Birk 1,2, I. Upmann 1, D. Toomre 3,
Focal switching of photochromic fluorescent proteins enables multiphoton microscopy with superior image contrast
 Focal switching of photochromic fluorescent proteins enables multiphoton microscopy with superior image contrast Ya-Ting Kao, Xinxin Zhu, Fang Xu, and Wei Min * Department of Chemistry, Columbia University,
Focal switching of photochromic fluorescent proteins enables multiphoton microscopy with superior image contrast Ya-Ting Kao, Xinxin Zhu, Fang Xu, and Wei Min * Department of Chemistry, Columbia University,
Journal of Molecular and Cellular Cardiology
 Journal of Molecular and Cellular Cardiology 58 (2013) 13 21 Contents lists available at SciVerse ScienceDirect Journal of Molecular and Cellular Cardiology journal homepage: www.elsevier.com/locate/yjmcc
Journal of Molecular and Cellular Cardiology 58 (2013) 13 21 Contents lists available at SciVerse ScienceDirect Journal of Molecular and Cellular Cardiology journal homepage: www.elsevier.com/locate/yjmcc
F luorescence imaging of live cells plays a crucial role in the study of biological processes at the cellular and
 SUBJECT AREAS: SUPER-RESOLUTION MICROSCOPY WIDE-FIELD FLUORESCENCE MICROSCOPY BIOPHOTONICS SINGLE-MOLECULE BIOPHYSICS Received 6 December 2012 Accepted 4 January 2013 Published 4 February 2013 Correspondence
SUBJECT AREAS: SUPER-RESOLUTION MICROSCOPY WIDE-FIELD FLUORESCENCE MICROSCOPY BIOPHOTONICS SINGLE-MOLECULE BIOPHYSICS Received 6 December 2012 Accepted 4 January 2013 Published 4 February 2013 Correspondence
Assembly of synapses by neuronal adhesion molecules: single molecule studies
 Assembly of synapses by neuronal adhesion molecules: single molecule studies Olivier Thoumine Interdisciplinary Institute of Neurosciences CNRS - University of Bordeaux Connectivity in the brain 300 nm
Assembly of synapses by neuronal adhesion molecules: single molecule studies Olivier Thoumine Interdisciplinary Institute of Neurosciences CNRS - University of Bordeaux Connectivity in the brain 300 nm
Protein localization in electron micrographs using fluorescence nanoscopy
 Nature Methods Protein localization in electron micrographs using fluorescence nanoscopy Shigeki Watanabe, Annedore Punge, Gunther Hollopeter, Katrin I Willig, Robert John Hobson, M Wayne Davis, Stefan
Nature Methods Protein localization in electron micrographs using fluorescence nanoscopy Shigeki Watanabe, Annedore Punge, Gunther Hollopeter, Katrin I Willig, Robert John Hobson, M Wayne Davis, Stefan
Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm
 Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm Yina Wang, 1,2,5 Joerg Schnitzbauer, 3,5 Zhe Hu, 1,2,5 Xueming Li, 4 Yifan Cheng,
Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm Yina Wang, 1,2,5 Joerg Schnitzbauer, 3,5 Zhe Hu, 1,2,5 Xueming Li, 4 Yifan Cheng,
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Contents. 11 The Use of Epitope Tags in Histochemistry References... 98
 Contents 1 Antibodies for Immunohistochemistry... 1 1.1 Structure of Antibodies... 2 1.2 Polyclonal Antibodies... 4 1.3 Mouse Monoclonal Antibodies... 4 1.4 Rabbit Monoclonal Antibodies... 5 1.5 Protein
Contents 1 Antibodies for Immunohistochemistry... 1 1.1 Structure of Antibodies... 2 1.2 Polyclonal Antibodies... 4 1.3 Mouse Monoclonal Antibodies... 4 1.4 Rabbit Monoclonal Antibodies... 5 1.5 Protein
DNA Microarray Technology
 2 DNA Microarray Technology 2.1 Overview DNA microarrays are assays for quantifying the types and amounts of mrna transcripts present in a collection of cells. The number of mrna molecules derived from
2 DNA Microarray Technology 2.1 Overview DNA microarrays are assays for quantifying the types and amounts of mrna transcripts present in a collection of cells. The number of mrna molecules derived from
Challenges to measuring intracellular Ca 2+ Calmodulin: nature s Ca 2+ sensor
 Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
DNA AND CHROMOSOMES. Genetica per Scienze Naturali a.a prof S. Presciuttini
 DNA AND CHROMOSOMES This document is licensed under the Attribution-NonCommercial-ShareAlike 2.5 Italy license, available at http://creativecommons.org/licenses/by-nc-sa/2.5/it/ 1. The Building Blocks
DNA AND CHROMOSOMES This document is licensed under the Attribution-NonCommercial-ShareAlike 2.5 Italy license, available at http://creativecommons.org/licenses/by-nc-sa/2.5/it/ 1. The Building Blocks
Imaging facilities at WUR
 Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology
 Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Cellular imaging using Nano- Materials. A Case-Study based approach Arun Murali, Srivats V
 Cellular imaging using Nano- Materials A Case-Study based approach Arun Murali, Srivats V Agenda Discuss a few papers Explain a couple of new imaging techniques and their benefits over conventional imaging
Cellular imaging using Nano- Materials A Case-Study based approach Arun Murali, Srivats V Agenda Discuss a few papers Explain a couple of new imaging techniques and their benefits over conventional imaging
Gene Expression Technology
 Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Far-field Light Microscopy
 Far-field Light Microscopy Christoph G Cremer, University of Heidelberg, Heidelberg, Germany Far-field light microscopy has reemerged as one of the major tools for studying the structure and dynamics of
Far-field Light Microscopy Christoph G Cremer, University of Heidelberg, Heidelberg, Germany Far-field light microscopy has reemerged as one of the major tools for studying the structure and dynamics of
The role of erna in chromatin looping
 The role of erna in chromatin looping Introduction Enhancers are cis-acting DNA regulatory elements that activate transcription of target genes 1,2. It is estimated that 400 000 to >1 million alleged enhancers
The role of erna in chromatin looping Introduction Enhancers are cis-acting DNA regulatory elements that activate transcription of target genes 1,2. It is estimated that 400 000 to >1 million alleged enhancers
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
Chapter 1. A Preview of the Cell. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 1 A Preview of the Cell Lectures by Kathleen Fitzpatrick Simon Fraser University The Cell Theory: A Brief History Robert Hooke (1665) observed compartments in cork, under a microscope, and first
Chapter 1 A Preview of the Cell Lectures by Kathleen Fitzpatrick Simon Fraser University The Cell Theory: A Brief History Robert Hooke (1665) observed compartments in cork, under a microscope, and first
JOURNAL OF NEUROCHEMISTRY doi: /jnc.12243
 JOURNAL OF NEUROCHEMISTRY 2013 126 203 212 doi: 10.1111/jnc.12243, *Department of Nanobiophotonics, Max Planck Institute for Biophysical Chemistry, G ottingen, Germany Weatherall Institute of Molecular
JOURNAL OF NEUROCHEMISTRY 2013 126 203 212 doi: 10.1111/jnc.12243, *Department of Nanobiophotonics, Max Planck Institute for Biophysical Chemistry, G ottingen, Germany Weatherall Institute of Molecular
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/22550 holds various files of this Leiden University dissertation. Author: Yan, Kuan Title: Image analysis and platform development for automated phenotyping
Cover Page The handle http://hdl.handle.net/1887/22550 holds various files of this Leiden University dissertation. Author: Yan, Kuan Title: Image analysis and platform development for automated phenotyping
