Green-to-red photoconvertible fluorescent proteins: tracking cell and protein dynamics on standard wide-field mercury arc-based microscopes
|
|
|
- Harriet Russell
- 6 years ago
- Views:
Transcription
1 METHODOLOGY ARTICLE Open Access Green-to-red photoconvertible fluorescent proteins: tracking cell and protein dynamics on standard wide-field mercury arc-based microscopes Susan M Baker 1,2, Robert W Buckheit III 1,3, Matthias M Falk 1* Abstract Background: Green fluorescent protein (GFP) and other FP fusions have been extensively utilized to track protein dynamics in living cells. Recently, development of photoactivatable, photoswitchable and photoconvertible fluorescent proteins (PAFPs) has made it possible to investigate the fate of discrete subpopulations of tagged proteins. Initial limitations to their use (due to their tetrameric nature) were overcome when monomeric variants, such as Dendra, meos, and mkikgr were cloned/engineered. Results: Here, we report that by closing the field diaphragm, selective, precise and irreversible green-to-red photoconversion ( nm illumination) of discrete subcellular protein pools was achieved on a wide-field fluorescence microscope equipped with standard DAPI, Fluorescein, and Rhodamine filter sets and mercury arc illumination within 5-10 seconds. Use of a DAPI-filter cube with long-pass emission filter (LP420) allowed the observation and control of the photoconversion process in real time. Following photoconversion, living cells were imaged for up to 5 hours often without detectable phototoxicity or photobleaching. Conclusions: We demonstrate the practicability of this technique using Dendra2 and meos2 as monomeric, photoconvertible PAFP representatives fused to proteins with low (histone H2B), medium (gap junction channel protein connexin 43), and high (a-tubulin; clathrin light chain) dynamic cellular mobility as examples. Comparable efficient, irreversible green-to-red photoconversion of selected portions of cell nuclei, gap junctions, microtubules and clathrin-coated vesicles was achieved. Tracking over time allowed elucidation of the dynamic live-cycle of these subcellular structures. The advantage of this technique is that it can be performed on a standard, relatively inexpensive wide-field fluorescence microscope with mercury arc illumination. Together with previously described laser scanning confocal microscope-based photoconversion methods, this technique promises to further increase the general usability of photoconvertible PAFPs to track the dynamic movement of cells and proteins over time. Background Expression of GFP-fusion proteins in live cells revolutionized cell biology by allowing for visualization and tracking of proteins of interest in real time at high spatio-temporal resolution. A few years ago, encouraged by the successful use of GFP and of other fluorescent proteins, several laboratories began to develop photoactivatable and photoconvertible fluorescent proteins (PAFPs), * Correspondence: mfalk@lehigh.edu 1 Department of Biological Sciences, Lehigh University, 111 Research Drive, Iacocca Hall, Bethlehem, PA 18015, USA which typically undergo a pronounced increase or shift in their spectral emission properties in response to UVviolet ( nm) or, in case of Dendra2 also intense blue light (488 nm) illumination [1-4]. Monomeric Anthozoa-derived green-to-red photoconvertible fluorescent proteins (Dendra2, meos2, mkikgr) showed particular promise for improved methods of tracking the dynamics of discrete protein pools within cells [5-8]. In the dark, these proteins mature to a green fluorescent state with a half-time of about 90 minutes at 37 C [7,9], while irradiation with UV-violet light (and also intense blue light in case of Dendra2) results in their irreversible 2010 Baker et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
2 Page 2 of 10 transition into a very photostable, bright-red fluorescent state that can be tracked for hours and days without significant photobleaching on a fluorescence microscope [6,7,10-12]. Protein kinetics and rates of protein exchange are typically determined through the use of techniques such as fluorescence recovery after photobleaching (FRAP) or fluorescence loss in photobleaching (FLIP). However, these techniques are limited in that photobleached protein populations may not be tracked beyond the point of photobleaching. Obviously, photoconversion offers a distinct advantage over photobleaching, if continuous tracking of subpopulations of tagged proteins is desired. Over the past decade, we have used green fluorescent protein (GFP) fusions and other color variants to track the dynamics of gap junction (GJ) channels in living cells, including by FRAP and FLIP techniques [13-19]. GJ channels assemble from hexamers of the four-pass transmembrane proteins called connexins (Cxs) that cluster together into so-called plaques in the lateral plasma membranes of cells to provide direct cell-to-cell communication and physical cell-cell coupling (Figure 1A, panel 1). GJ plaques can be aligned in two principal orientations: perpendicular to the image plane, providing a view onto their edge (GJ plaques circled in the center of Figure 1A and shown in Figures 1B, C); or horizontally, if cells grow partially on top of each other, providing a view onto their surface (en face) (GJ plaques circled on the right of Figure 1A and shown in the remaining panels of Figure 1A). A typical GJ plaque can consist of hundreds to thousands of densely packed individual channels. GJs are surprisingly dynamic membrane structures. Their structural connexin proteins have been found to turn over with a half-life of only 1 to 5 hours [15,20-22]. Tagging of Cx-proteins with tetrameric photoconvertible fluorescent proteins, such as DsRed was found not to be feasible, probably due to the disruption of appropriate Cx-oligomerization by the tetrameric FP tag [13,23]. However, monomeric fluorescent protein tags such as GFP, mcherry, meos2, or Dendra2 are tolerated by many different proteins without detectable interference with their function, including Cxs [3,4,6,7,10,14,15,24-26]. We report an efficient technique that makes photoconversion and tracking of discrete protein pools feasible on simpler, less expensive mercury arc-based fluorescence microscopes equipped with standard fluorescence filter sets that complements previously published more sophisticated laser scanning confocal microscope-based techniques [3,4,6,7,10,12,26,27]. We demonstrate the feasibility of this technique using Dendra2-tagged histone H2B (Dendra2-H2B), connexin43 (Cx43-Dendra2), a-tubulin (Dendra2-a-tubulin), and clathrin light chain (meos2-clathrin light chain) as examples of proteins exhibiting a wide array of dynamic properties. A detailed step-by-step photoconversion protocol is provided. Results and Discussion To demonstrate the feasibility of photoconverting and tracking discrete subcellular PAFP-tagged protein pools on a wide-field fluorescence microscope, we generated and used cdna constructs in which Dendra2 or meos2, two available comparable monomeric PAFPs, were fused to the C-terminus of the GJ protein Cx43, or to the N-termini of histone H2B, a-tubulin and clathrin light chain [4,15,26]. In transiently transfected HeLa cells, we observed expression, assembly, trafficking and localization of all four fusion-protein constructs similar to untagged, or GFP-tagged fusion constructs as reported previously [4,14,25,26] (Figures 1, 2, 3 and 4). Using nm mercury arc lamp illumination (standard FITC or GFP-filter sets), significantly attenuated by neutral density filters (ND8/ND4), we were able to visualize and image green Dendra2- and meos2-fluorescence without unintentionally photoconverting these PAFPs from green to red fluorescence (Figures 1, 2, 3 and 4). Dendra2/mEos2 fusion-protein pools underwent precise and selective photoconversion by moving the target areas to the center of the visible field, closing the field diaphragm to pinhole size (exposing an area as small as 20 μm diameter using a 100 objective), and exposing the areas to full, nm illumination (standard DAPI filter set) for 5-10 seconds. Use of a DAPI-filter cube with 420 nm long-pass emission filter (LP420) allowed to observe and manipulate the photoconversion process in real time. Following photoconversion, filters were switched to the red fluorescence filter cube (standard Rhodamine filter set; excitation: nm; emission: nm), the field diaphragm was re-opened completely, and the red photoconverted Dendra2 signal was detected and imaged using the red filter cube. Depending on exposure time, up to 90% of the green Dendra2/mEos2 fluorescence in the target area was photoconverted into red fluorescence within a few seconds (Figures 1, 2, 3 and 4). Green fluorescence outside thetargetareawasnotconverted(figures1,2,3and 4). Subsequent to photoconversion, cells were imaged repeatedly in both green (attenuated by neutral density filters to prevent further unintentional photoconversion) and red channels at indicated time intervals for up to 5 hours (Figures 1, 2, 3 and 4). Over a 5-hour period, in live cells expressing Cx43- Dendra2, we observed a significant loss of red fluorescence from the target area and recovery of the green fluorescence signal (Figure 1B), indicating the continuous removal of older, red photoconverted Cx43-Dendra2-based GJ channels and delivery of newly synthesized, green Cx43-Dendra2 channels to GJ
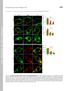
3 Baker et al. BMC Cell Biology 2010, 11:15 Page 3 of 10 Figure 1 Gap junction (GJ) dynamics revealed by photoconverting expressed Cx43-Dendra2. (A) GJs consisting of many densely packed channels visible as green lines and puncta between HeLa cells expressing Cx43-Dendra2 (panel 1). GJs orient perpendicular providing a view onto their edge (circled in center, panel 1; also shown in Figures 1B, C), or horizontally providing a view onto their surface (circled on right; remaining panels, Figure 1A). GJs are dynamic structures. Their channels are replaced within several hours, as demonstrated by photoconverting Dendra2-tagged Cx43. A region containing two horizontally oriented GJs was photoconverted (entire field shown), and green and red channels were recorded over time. Within 1-hour post conversion a widening, homogenous green line of channels appeared along the GJs (panels 2-5). (B) Following photoconverted GJs for longer periods resulted in a steady loss of red fluorescence from the photoconverted area, and a simultaneous recovery of green fluorescence (circled in panel 1), suggesting that older channels are continuously removed from central GJ areas, while newly synthesized channels are simultaneously added to their periphery. Fluorescence intensity profiles for red and green channels measured along lines traversing the photoconverted GJs are shown. (C) Photoconversion allows estimation of GJ channel turnover. A portion of a perpendicular oriented Cx43-Dendra2 GJ was photoconverted (circled). Over time, red fluorescent puncta appeared adjacent to the converted GJ area (arrow-heads, panels 3, 4). Puncta were not detected immediately post-conversion (panel 2), suggesting that they were released from the photoconverted GJ area. Puncta correspond to degradative endocytic vesicles that are generated by the release of small GJ channel packets from GJs [15]. (D) Schematic representation of GJ turnover as shown experimentally in (A-C).
4 Page 4 of 10 Figure 2 Histone dynamics revealed by photoconverting expressed Dendra2-H2B. Entire cell nuclei (in A, and top row in B), or portions of nuclei (center and bottom rows in B) of Dendra2-H2B expressing HeLa cells were photoconverted within 5-10 sec (circled areas), and green and red channels were imaged immediately after photoconversion (in A), and after 1-hour (in B). One-hour post conversion, the areas of photoconverted histone H2B protein and the edges between photoconverted and unconverted Dendra2-H2B domains were still well defined, revealing H2B s stable association with DNA in interphase chromatin. DIC and fluorescence images were merged in (A) to reveal the location of cell nuclei, and transiently transfected Dendra2-H2B expressing cells. Note that more or less efficient photoconversion of cell nuclei was achieved (compare remaining green fluorescence in the circled areas in B, rows 1 and 3, compared with row 2).
5 Baker et al. BMC Cell Biology 2010, 11:15 Page 5 of 10 Figure 3 Microtubule dynamics revealed by photoconverting expressed Dendra2-a-tubulin. A distal portion of a Dendra2-a-tubulin expressing HeLa cell was photoconverted within 5 sec (entire field shown in the panels). Note, that photoconversion was only incomplete, as indicated by the relatively strong remaining green fluorescence (visible in the left panel of row 2). Within 3-7 minutes, red photoconverted and remaining green a-tubulin pools intermixed (arrows), consistent with the known dynamic continuous polimerization and depolimerization of microtubules, and the diffusional mobility of unassembled a/b-tubulin subunit-dimers in the cytoplasm.
6 Page 6 of 10 Figure 4 Clathrin-coated vesicle dynamics revealed by photoconverting expressed meos2-clathrin light chain. Numerous clathrin-coated vesicles are visible in the cytoplasm of an meos2-clathrin light chain expressing HeLa cell (panel 1, row 1). A distal portion of this vesicle pool was photoconverted within about 10 sec (circled). Within 2-5 minutes after photoconversion, photoconverted and remaining, unconverted vesicles moved laterally and intermixed (red, green and yellow vesicles, the resulting color when red and green fluorescence colocalize, arrows), consistent with the known dynamic mobility and structural composition of these vesicles. N = Cell nucleus. plaques (Figure 1D), as observed previously for GJs using other fluorescence based techniques [15,17,28]. To confirm that photoconversion was irreversible and stable, and we were not observing theoretical autorecovery of green Dendra2-fluorescence, we fixed Cx43- Dendra2 expressing cells in formaldehyde following photoconversion. No recovery of green fluorescence was observed hours, or even days post conversion (not shown). To track accrual of new channels to GJ plaques, a 20 μm diameter region (using a 100 objective and maximally closed field-diaphragm) encircling two Cx43-Dendra2 GJ plaques was photoconverted from green to red fluorescence (Figure 1A, panel 2, 3) and imaged every 30 minutes for 2 hours. After 1 hour, a distinct rim of newly accrued (green) channels had appeared along the edges of the photoconverted (red) GJ plaques (Figure 1A, panel 4) that became wider and more intense over
7 Page 7 of 10 time (Figure 1A, right panel), indicating that new channels are indeed accrued along the outer edges of GJ plaques, while older channels are simultaneously removed from plaque centers as schematically shown in Figure 1D and in references [15,17]. These findings are consistent with what has previously been reported for channel accrual to GJ plaques using fluorescence recovery after photobleaching (FRAP) and successive FlAsH and ReAsH labeling techniques [17,28]. To investigate whether this technique would allow estimation of protein turnover kinetics, a portion of a perpendicular oriented Cx43-Dendra2 GJ plaque was photoconverted from green to red fluorescence as described above (Figure 1C, left panel, circled area) and imaged every 5 minutes. Over time, we detected red fluorescent puncta in the cytoplasm adjacent to photoconverted plaques that accumulated over the 15 min time period (Figure 1C, panels 3, 4, marked with arrowheads). The red fluorescent puncta we recognized as degradative endocytic vesicles that were released from the photoconverted GJ plaque area and later were degraded by lysosomal pathways [15] (not shown). These red fluorescent vesicles were not detected immediately post-photoconversion (Figure 1C, panel 2), suggesting that they were not already present in the cytoplasm at the time of photonversion. Calculating surface areas of released vesicles revealed a half-life of ~2.5 hours (approximately 10 μm 2 of a 50 μm 2 photoconverted GJ plaque area was internalized in one hour) [15] that falls within the estimated half-life of 1-5 hours reported previously for GJs [20-22]. While certainly possible on wide-field microscopes, quantitative photoconversion experiments depending on the target, may however better be performed on confocal microscope systems, that allow to precisely restrict the photoconversion laser beam to the target area without potentially photoconverting unintentionally other structures, that are also located within the circular wide-field microscope photoconversion area [15]. Histones H2A, H2B, H3, and H4 are the core protein components of nucleosomes and are known to be bound stably to interphase chromatin; and this was confirmed by the appearance of the photoconverted red Dendra2-histone-H2B fluorescence that stayed locally unchanged over time in the cell nuclei of living, Dendra2-histone-H2B-expressing HeLa cells imaged before, immediately after, and 1 hour after photoconversion (Figures 2A, B). In contrast, clathrin and tubulin are known to be highly dynamic cellular proteins and this was confirmed in our photoconversion experiments. Microtubules are assembled from a/b-tubulin dimers and both populations, assembled microtubulesaswellasunassembled cytoplasmically located tubulin dimers (diffuse green fluorescence), are visible in living Dendra2-a-tubulin expressing Hela cells (Figure 3, top row). In addition, microtubules are known to constantly grow and shrink, resulting in a dynamic exchange of subunits in an assembled microtubule. Within a few minutes after a distal portion of a Dendra2-a-tubulin expressing HeLa cell was partially photoconverted (Figure 3, second row, arrows), red photoconverted and remaining green a-tubulin pools were observed to intermix, consistent with the known dynamic growth behavior of microtubules, and the mobility of unassembled subunits in the cytoplasm. Tubulin dynamics resulted in a diffusion of red fluorescence away from the distal, photoconverted area and an increasing re-occurrence of unconverted green a-tubulin in the distal cell area within several minutes after photoconversion (Figure 3, rows 3, 4). Clathrin triskelions consisting of three light and three heavy chains assemble into flat, and curved lattices on the plasma membrane to build the coats of endocytic vesicles. Numerous such vesicles are visible in living HeLa cells expressing meos2-clathrin light chain (Figure 4, top row). Within 5 minutes after a distal portion of this vesicle pool in a cell was successfully photoconverted (circled in Figure 4, panel 1; rows 2-4, arrows), red photoconverted and remaining green vesicles were observed to move laterally and to intermix with each other, consistent with the dynamic nature of these vesicles that are trafficked throughout the cell along microtubules (Figure 4, bottom row). While several studies have described photoconversion of PAFPs using laser scanning confocal microscopy [6,7,9-11,15,26], we describe here PAFP-photoconversion of discrete protein pools using a simpler, less expensive mercury arc-based microscope system. As such, there are restrictions presented by photoconverting PAFPs on a standard wide-field fluorescence microscope as opposed to a laser scanning confocal microscope system. First, the smallest region that can be photoconverted is limited in size and shape to a circular area of about 20 μm (using a 100 objective; about 35 μm usinga60 objective) that is defined by the minimal remaining opening of the field diaphragm. However, aftermarket 10 μm diameter pinhole- and slit-sliders are available to partially overcome this limitation (e.g. slit/pinhole slider for Olympus inverted microscopes; Melles Griot Inc., Albuquerque, NM, USA). Since photon emission of mercury arc bulbs is less intense compared to lasers, photoconversion on mercury-arc lamp based microscope systems takes longer (several seconds at UV-violet excitation), and photoconversion might remain incomplete (Figures 1, 2, 3 and 4). Prolonged exposure to short-wavelength light can be detrimental to living cells that may react by inducing apoptosis in response to toxic short-wavelength
8 Page 8 of 10 illumination (e.g. DAPI nm band-pass excitation filter). Thus, the addition of HEPES-buffer and Oxyrase (Oxyrase Inc., Mansfield, Ohio, USA) to the cell-culture medium to degrade toxic oxygen radicals generated during short-wavelength excitation is recommended. Also, Dendra2 and meos2 s major photoconversion wavelength is 405 nm [7,26] and optical filters allowing specific excitation at this red-shifted, less harmful wavelength are commercially available (Chroma, Omega, Semrock). On the other hand, due to the less intense mercury arc lamp illumination, photobleaching instead of photoconversion that will occur on confocal microscope systems if laser power is set too high is not problematic. Furthermore, Dendra2 (but not meos2) has a second less efficient photoconversion peak at 490 nm [7,26] that renders green Dendra2 sensitive to intense blue light; and allows Dendra2 (but not meos2) to be photoconverted on standard Argon-Helium/Neon laserequipped confocal microscopes. Thus, when imaging green Dendra2, illumination attenuated by a neutral density filter (or very low 488 nm laser power) is required to avoid inadvertent photoconversion. However, if phototoxicity due to UV-violet illumination should be a problem, less efficient, but marginally toxic un-attenuated FITC-filter illumination ( nm excitation band pass filter) can be used to photoconvert Dendra2, instead of using the DAPI filter set. Conclusions We demonstrate here efficient and irreversible photoconversion of discrete subcellular protein pools of cells grown in culture on wide-field fluorescence microscopes, equipped with standard filter cubes and mercury arc-lamp illumination, using several Dendra2- and meos2-tagged proteins with a wide variety of dynamic properties as examples. Dendra2 and meos2 behaved comparable in respect of time required for photoconversion (5-10 sec), and conversion efficiency (30-90%). Photobleaching during photoconversion, and afterwards during repeated imaging, was not observed as being problematic. The advantage of this method is its greater simplicity requiring easier to use, less expensive microscope systems that makes this technique especially appealing to less well equipped institutions, for instructional and teaching purposes, and to applications where defining a region of interest of specific size or shape (e.g. whole cell applications) is not required. Disadvantages to laser scanning confocal microscope-based photoconversion techniques include the limited control of the region to be photoconverted (circular, 20 μm in diameter), and longer, potentially more toxic UV-violet photoconversion times. Together with previously described laser scanning confocal microscope-based photoconversion methods, this technique promises to further increase the general usability of photoconvertible PAFPs to track the dynamic movement of cells and proteins over time. This technique may also be applicable to locally uncage fluorescent probes, activate pagfp, or to study the effects of locally induced photo-damage. Methods Plasmid construction for expression in mammalian cells For expression in eukaryotic cells, a BamHI-EcoRI fragment of cdna encoding Cx43 described in ref [14] was inserted into the pdendra2-n1 plasmid. The cdna endcoding Cx43 was obtained from a BamH1-EcoRI digest of a Cx43-pEGFP-N1 plasmid. The pdendra2-n1 vector, the Dendra2-H2B, the Dendra2-human a-tubulin, and the meos2-clathrin light chain constructs were generous gifts of Michael W. Davidson (Florida State University, Tallahassee, FL). Cell culture and transfection HeLa cells (CCL 2, American Type Culture Collection, Manassas, VA) were cultured under standard culture conditions as described previously [14]. For all experiments, cells were grown on 35 mm diameter glass bottomed culture dishes coated with 20 μg/ml collagen (Mat-Tek Corp., Ashland, MA). Cells were transfected using Superfect transfection reagent (Qiagen, Valencia, CA) according to manufacturer s recommendations 24 hours prior to photoconversion experiments. All photoconversion experiments were carried out at 37 C in standard culture medium supplemented with HEPES (15 μg/ml) (Sigma) and oxyrase (40 μl/ml) (Oxyrase Inc.) in an enclosed environmental control chamber enclosing the microscope. Fluorescence microscopy and photoconversion Time-lapse microscopy was performed on a Nikon Eclipse TE 2000E inverted fluorescence microscope equipped as previously described [19]. Photoconversion was performed by reducing the field diaphragm to pinhole size, at 100 magnification with near-uv irradiation ( nm) for 5-10 seconds. Photoconversion was monitored in real time using a DAPI filter cube with long-path emission filter (LP420). Similarly efficient photoconversion (30-90%) was observed with both Dendra2 and meos2 PAFPs. Images were captured and analyzed using MetaVue software version 6.1r5 (Molecular Devices, Sunnyvale, CA) and processed using Adobe Photoshop (Adobe Systems, Mountain View, CA). Unintentional photoconversion by room-light was not observed to be problematic, and cells -previous to photoconversion- where handled under standard cell culture conditions (e.g. dishes were not wrapped in aluminum foil).
9 Page 9 of 10 Step-by-step procedure to photoconvert Dendra2- and meos2-tagged protein pools on wide-field fluorescence microscopes Steps to be done before photoconversion 1) Darken the room completely and dark-adapt your eyes. 2) Rotate filter cubes to blue light excitation position (FITC filter cube). 3) Push neutral density filters into light path. 4) Close excitation shutter on the microscope. 5) Rotate 40, 60, or 100 oil immersion objective into position and add immersion oil. 6) Place dish with Dendra2/mEos2-expressing cells on the stage. 7) Focus cells using white-light Phase Contrast or DIC illumination. 8) Open excitation shutter and search for expressing cells using strongly attenuated light only! 9) Acquire an image with attenuated blue excitation light (pre-conversion green image). 10) Rotate filter cubes to green light excitation position (TRITC filter cube), pull out neutral density filters and acquire another image (pre-conversion red image). There should be no, or only minimal red Dendra2-emission signal visible. If there is a strong red signal visible, accidental photoconversion of the field of few has already occurred, and a new region of interest needs to be selected. 11) Push neutral-density filters back into the light path. For photoconversion the following steps need to be performed 12) Rotate filter cubes to the DAPI cube position (UV excitation), pull out neutral density filters and watch the photoconversion process through the oculars (developing red, photoconverted Dendra2/mEos2-emission is visible if DAPI cube is equipped with 420 nm long path emission filter). This takes about 5-10 seconds to achieve 30-90% efficient photoconversion. 13) Push neutral density filters back into the light pass to end the photoconversion process. 14) Acquire post-conversion images with blue (FITC cube) and red (TRITC cube) light illumination under the same conditions used for pre-conversion images. A strong red Dendra2-emission signal should now be visible. 15) To restrict photoconversion to the center of the field of view, place region of interest in the center of the viewable field. Close the field diaphragm to the desired position before photoconversion. Photoconvert region of interest. Re-open field diaphragm after photoconversion has been terminated. 16) Acquire green and red post-conversion images at desired time intervals under the conditions described above. 17) Merge green and red post-conversion images if desired. Abbreviations Cx: connexin; FLIP: fluorescence loss in photobleaching; FRAP: fluorescence recovery after photobleaching; GFP: green fluorescent protein; GJ: gap junction; LP filters: long pass filters; ND filters; neutral density filters; PAFP: photoactivatable fluorescent proteins; UV: ultra-violet. Acknowledgements We thank Michael W. Davidson (State University of Florida, Tallahassee, FL) for generously providing vectors and plasmids, Anna Gumpert for help with line-scans, and Lehigh University s BioS368 Cell Biology Laboratory students for generating photoconversion images of Dendra2-histone H2B shown in Figure 2A. This work was supported by National Institutes of Health, National Institute of General Medical Sciences, grant GM to MMF. Author details 1 Department of Biological Sciences, Lehigh University, 111 Research Drive, Iacocca Hall, Bethlehem, PA 18015, USA. 2 Current address: National Center for Forensic Science, University of Central Florida, Orlando, FL, USA. 3 Current address: Johns Hopkins School of Medicine, Cellular and Molecular Medicine Graduate Training Program, Baltimore, MD 21231, USA. Authors contributions MMF conceived and designed the study and the experiments; SMB, RWB, and MMF performed the experiments and analyzed the data; SMB and MMF drafted and wrote the manuscript and assembled and generated the figures. All authors read and approved the final manuscript. Received: 21 November 2009 Accepted: 22 February 2010 Published: 22 February 2010 References 1. Lukyanov KA, Chudakov DM, Lukyanov S, Verkhusha VV: Innovation: Photoactivatable fluorescent proteins. Nat Rev Mol Cell Biol 2005, 6(11): Muller-Taubenberger A, Anderson KI: Recent advances using green and red fluorescent protein variants. Appl Microbiol Biotechnol 2007, 77(1): Piston DW, Kremers G-J, Benninger RKP, Davidson MW: Photoactivation in fluorescence microscopy. Microscopy Today 2009, 17(4): Shaner NC, Patterson GH, Davidson MW: Advances in fluorescent protein technology. J Cell Sci 2007, 120(Pt 24): Chudakov DM, Lukyanov S, Lukyanov KA: Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol 2005, 23(12): Chudakov DM, Lukyanov S, Lukyanov KA: Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat Protoc 2007, 2(8): Gurskaya NG, Verkhusha VV, Shcheglov AS, Staroverov DB, Chepurnykh TV, Fradkov AF, Lukyanov S, Lukyanov KA: Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotechnol 2006, 24(4): Habuchi S, Tsutsui H, Kochaniak AB, Miyawaki A, van Oijen AM: mkikgr, a monomeric photoswitchable fluorescent protein. PLoS One 2008, 3(12): e Zhang L, Gurskaya NG, Merzlyak EM, Staroverov DB, Mudrik NN, Samarkina ON, Vinokurov LM, Lukyanov S, Lukyanov KA: Method for realtime monitoring of protein degradation at the single cell level. Biotechniques 2007, 42(4):446, 448, Chudakov DM, Lukyanov S, Lukyanov KA: Using photoactivatable fluorescent protein Dendra2 to track protein movement. Biotechniques 2007, 42(5):553, 555, 557 passim Mavrakis M, Rikhy R, Lippincott-Schwartz J: Plasma membrane polarity and compartmentalization are established before cellularization in the fly embryo. Dev Cell 2009, 16(1): Nowotschin S, Hadjantonakis AK: Use of KikGR a photoconvertible greento-red fluorescent protein for cell labeling and lineage analysis in ES cells and mouse embryos. BMC Dev Biol 2009, 9: Falk M: Genetic tags for labelling live cells: gap junctions and beyond. Trends Cell Biol 2002, 12(9): Falk MM: Connexin-specific distribution within gap junctions revealed in living cells. J Cell Sci 2000, 113(Pt 22):
10 Page 10 of Falk MM, Baker SM, Gumpert AM, Segretain D, Buckheit RW: Gap junction turnover is achieved by the internalization of small endocytic doublemembrane vesicles. Mol Biol Cell 2009, 20(14): Falk MM, Lauf U: High resolution, fluorescence deconvolution microscopy and tagging with the autofluorescent tracers CFP, GFP, and YFP to study the structural composition of gap junctions in living cells. Microsc Res Tech 2001, 52(3): Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM: Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci USA 2002, 99(16): Lopez P, Balicki D, Buehler LK, Falk MM, Chen SC: Distribution and dynamics of gap junction channels revealed in living cells. Cell Commun Adhes 2001, 8(4-6): Piehl M, Lehmann C, Gumpert A, Denizot JP, Segretain D, Falk MM: Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell 2007, 18(2): Beardslee MA, Laing JG, Beyer EC, Saffitz JE: Rapid turnover of connexin43 in the adult rat heart. Circ Res 1998, 83(6): Berthoud VM, Minogue PJ, Laing JG, Beyer EC: Pathways for degradation of connexins and gap junctions. Cardiovasc Res 2004, 62(2): Fallon RF, Goodenough DA: Five-hour half-life of mouse liver gapjunction protein. J Cell Biol 1981, 90(2): Lauf U, Lopez P, Falk MM: Expression of fluorescently tagged connexins: a novel approach to rescue function of oligomeric DsRed-tagged proteins. FEBS Lett 2001, 498(1): Bukauskas FF, Jordan K, Bukauskiene A, Bennett MV, Lampe PD, Laird DW, Verselis VK: Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proceedings of the National Academy of Sciences of the United States of America 2000, 97(6): Jordan K, Solan JL, Dominguez M, Sia M, Hand A, Lampe PD, Laird DW: Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Molecular Biology of the Cell 1999, 10(6): McKinney SA, Murphy CS, Hazelwood KL, Davidson MW, Looger LL: A bright and photostable photoconvertible fluorescent protein. Nat Methods 2009, 6(2): Subach FV, Subach OM, Gundorov IS, Morozova KS, Piatkevich KD, Cuervo AM, Verkhusha VV: Monomeric fluorescent timers that change color from blue to red report on cellular trafficking. Nat Chem Biol 2009, 5(2): Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH: Multicolor and electron microscopic imaging of connexin trafficking. Science 2002, 296(5567): doi: / Cite this article as: Baker et al.: Green-to-red photoconvertible fluorescent proteins: tracking cell and protein dynamics on standard wide-field mercury arc-based microscopes. BMC Cell Biology :15. Submit your next manuscript to BioMed Central and take full advantage of: Convenient online submission Thorough peer review No space constraints or color figure charges Immediate publication on acceptance Inclusion in PubMed, CAS, Scopus and Google Scholar Research which is freely available for redistribution Submit your manuscript at
Feb No. 22. Dmitriy Chudakov, Timo Zimmermann
 Feb. 2007 No. 22 Using photoactivatable fluorescent proteins Dendra2 and PS-CFP2 to track protein movement with Leica TCS SP5 Dmitriy Chudakov, Timo Zimmermann LAS AF: FRAP Wizard Live Data Mode Photoconversion
Feb. 2007 No. 22 Using photoactivatable fluorescent proteins Dendra2 and PS-CFP2 to track protein movement with Leica TCS SP5 Dmitriy Chudakov, Timo Zimmermann LAS AF: FRAP Wizard Live Data Mode Photoconversion
STORM/PALM. Super Resolution Microscopy 10/31/2011. Looking into microscopic world of life
 Super Resolution Microscopy STORM/PALM Bo Huang Department of Pharmaceutical Chemistry, UCSF CSHL Quantitative Microscopy, 1/31/211 Looking into microscopic world of life 1 µm 1 µm 1 nm 1 nm 1 nm 1 Å Naked
Super Resolution Microscopy STORM/PALM Bo Huang Department of Pharmaceutical Chemistry, UCSF CSHL Quantitative Microscopy, 1/31/211 Looking into microscopic world of life 1 µm 1 µm 1 nm 1 nm 1 nm 1 Å Naked
Fast, three-dimensional super-resolution imaging of live cells
 Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Rational design of true monomeric and bright photoconvertible
 Rational design of true monomeric and bright photoconvertible fluorescent proteins Mingshu Zhang, Hao Chang, Yongdeng Zhang, Junwei Yu, Lijie Wu, Wei Ji, Juanjuan Chen, Bei Liu, Jingze Lu, Yingfang Liu,
Rational design of true monomeric and bright photoconvertible fluorescent proteins Mingshu Zhang, Hao Chang, Yongdeng Zhang, Junwei Yu, Lijie Wu, Wei Ji, Juanjuan Chen, Bei Liu, Jingze Lu, Yingfang Liu,
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Localization Microscopy
 Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
HHS Public Access Author manuscript Nat Methods. Author manuscript; available in PMC 2009 November 01.
 Photoconversion in orange and red fluorescent proteins Gert-Jan Kremers 1, Kristin L. Hazelwood 2, Christopher S. Murphy 2, Michael W. Davidson 2, and David W. Piston 1 1 Department of Molecular Physiology
Photoconversion in orange and red fluorescent proteins Gert-Jan Kremers 1, Kristin L. Hazelwood 2, Christopher S. Murphy 2, Michael W. Davidson 2, and David W. Piston 1 1 Department of Molecular Physiology
Post-expansion antibody delivery, after epitope-preserving homogenization.
 Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
Special Techniques 1. Mark Scott FILM Facility
 Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Photoactivatable mcherry for high-resolution two-color fluorescence microscopy
 Photoactivatable mcherry for high-resolution two-color fluorescence microscopy Fedor V Subach 1 3, George H Patterson,3, Suliana Manley, Jennifer M Gillette, Jennifer Lippincott-Schwartz & Vladislav V
Photoactivatable mcherry for high-resolution two-color fluorescence microscopy Fedor V Subach 1 3, George H Patterson,3, Suliana Manley, Jennifer M Gillette, Jennifer Lippincott-Schwartz & Vladislav V
PALM/STORM, BALM, STED
 PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
Supplementary Material
 Supplementary Material Supplementary Table 1. Definitions of variants described in this work. Protein variant Substitutions and modifications relative to parent mtfp1 a = cfp484 b + delete all residues
Supplementary Material Supplementary Table 1. Definitions of variants described in this work. Protein variant Substitutions and modifications relative to parent mtfp1 a = cfp484 b + delete all residues
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
Confocal Microscopes. Evolution of Imaging
 Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
Fluorescence microscopy
 Fluorescence microscopy 1 Fluorescence microscopies basic fluorescence, fluorophores Deconvolution Confocal Two-photon/multi-photon 4Pi Light sheet Total internal reflection STED FRAP/FLIP/FCS FRET PALM/STORM/iPALM
Fluorescence microscopy 1 Fluorescence microscopies basic fluorescence, fluorophores Deconvolution Confocal Two-photon/multi-photon 4Pi Light sheet Total internal reflection STED FRAP/FLIP/FCS FRET PALM/STORM/iPALM
Live cell microscopy
 Live cell microscopy 1. Why do live cell microscopy? 2. Maintaining living cells on a microscope stage. 3. Considerations for imaging living cells. 4. Fluorescence labeling of living cells. 5. Imaging
Live cell microscopy 1. Why do live cell microscopy? 2. Maintaining living cells on a microscope stage. 3. Considerations for imaging living cells. 4. Fluorescence labeling of living cells. 5. Imaging
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit
 Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Microscopy from Carl Zeiss. DirectFRAP. News from the Cell. The New Class of Laser Manipulation for the Analysis of Cell Dynamics
 Microscopy from Carl Zeiss DirectFRAP News from the Cell The New Class of Laser Manipulation for the Analysis of Cell Dynamics DirectFRAP. New Insights into Cell Dynamics. Fluorescence breaks new ground:
Microscopy from Carl Zeiss DirectFRAP News from the Cell The New Class of Laser Manipulation for the Analysis of Cell Dynamics DirectFRAP. New Insights into Cell Dynamics. Fluorescence breaks new ground:
Dino-Lite knowledge & education. Fluorescence Microscopes
 Dino-Lite knowledge & education Fluorescence Microscopes Dino-Lite Fluorescence models Smallest fluorescence microscope in the world Revolution to biomedical and educational applications Flexible Easy
Dino-Lite knowledge & education Fluorescence Microscopes Dino-Lite Fluorescence models Smallest fluorescence microscope in the world Revolution to biomedical and educational applications Flexible Easy
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
What to look for in a fluorophore. What to do with a fluorophore. Types of fluorochromes
 What to do with a fluorophore Intracellular localization (ER, Golgi, PM, nuclear, lysosome, MT, actin,...) Dynamic processes (protein synthesis, trafficking, turnover, DNA replication, cytoskeletal remodeling,
What to do with a fluorophore Intracellular localization (ER, Golgi, PM, nuclear, lysosome, MT, actin,...) Dynamic processes (protein synthesis, trafficking, turnover, DNA replication, cytoskeletal remodeling,
Caspase-2 BiFC plasmids
 Caspase-2 BiFC plasmids Caspase-2 BiFC - background Bimolecular Fluorescence Complementation (BiFC) describes the use of split fluorescent proteins to measure protein-protein interactions in cells. The
Caspase-2 BiFC plasmids Caspase-2 BiFC - background Bimolecular Fluorescence Complementation (BiFC) describes the use of split fluorescent proteins to measure protein-protein interactions in cells. The
Tracking Cellular Protein Localization and Movement in Cells with a Flexible Fluorescent Labeling Technology. Chad Zimprich January 2015
 Tracking Cellular Protein Localization and Movement in Cells with a Flexible Fluorescent Labeling Technology Chad Zimprich January 2015 Presentation verview HaloTag Fusion Technology Design Functionality
Tracking Cellular Protein Localization and Movement in Cells with a Flexible Fluorescent Labeling Technology Chad Zimprich January 2015 Presentation verview HaloTag Fusion Technology Design Functionality
Photoactivatable. Fluorescent Proteins. Effective tool to monitor cellular events in real time
 Section B Photoactivatable Fluorescent Proteins Effective tool to monitor cellular events in real time t r a c k i n g c e l l m o v e m e n t m o n i t o r i n g c o m m u n i c a t i o n p a t h w a
Section B Photoactivatable Fluorescent Proteins Effective tool to monitor cellular events in real time t r a c k i n g c e l l m o v e m e n t m o n i t o r i n g c o m m u n i c a t i o n p a t h w a
Supplementary Information. A superfolding Spinach2 reveals the dynamic nature of. trinucleotide repeat RNA
 Supplementary Information A superfolding Spinach2 reveals the dynamic nature of trinucleotide repeat RNA Rita L. Strack 1, Matthew D. Disney 2 & Samie R. Jaffrey 1 1 Department of Pharmacology, Weill Medical
Supplementary Information A superfolding Spinach2 reveals the dynamic nature of trinucleotide repeat RNA Rita L. Strack 1, Matthew D. Disney 2 & Samie R. Jaffrey 1 1 Department of Pharmacology, Weill Medical
Super-resolution Microscopy
 Semr oc kwhi t epaperser i es : 1. Introduction Super-resolution Microscopy Fluorescence microscopy has revolutionized the study of biological samples. Ever since the invention of fluorescence microscopy
Semr oc kwhi t epaperser i es : 1. Introduction Super-resolution Microscopy Fluorescence microscopy has revolutionized the study of biological samples. Ever since the invention of fluorescence microscopy
SUPPLEMENTARY INFORMATION
 a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
Partha Roy
 Fluorescence microscopy http://micro.magnet.fsu.edu/primer/index.html Partha Roy 1 Lecture Outline Definition of fluorescence Common fluorescent reagents Construction ti of a fluorescence microscope Optical
Fluorescence microscopy http://micro.magnet.fsu.edu/primer/index.html Partha Roy 1 Lecture Outline Definition of fluorescence Common fluorescent reagents Construction ti of a fluorescence microscope Optical
D e c N o. 2 8
 D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
More on fluorescence
 More on fluorescence Last class Fluorescence Absorption emission Jablonski diagrams This class More on fluorescence Common fluorophores Jablonski diagrams to spectra Properties of fluorophores Excitation
More on fluorescence Last class Fluorescence Absorption emission Jablonski diagrams This class More on fluorescence Common fluorophores Jablonski diagrams to spectra Properties of fluorophores Excitation
SI Appendix. Supporting Materials (references cited here can be found in the reference list in the main text):
 SI Appendix Supporting Materials (references cited here can be found in the reference list in the main text): SupplementaryText Figs. S1 to S13 Table S1 Captions for Supporting Movies S1 to S5 Other Supporting
SI Appendix Supporting Materials (references cited here can be found in the reference list in the main text): SupplementaryText Figs. S1 to S13 Table S1 Captions for Supporting Movies S1 to S5 Other Supporting
Transcriptional regulation of IFN-l genes in Hepatitis C virus-infected hepatocytes via IRF-3 IRF-7 NF- B complex
 POSTER PRESENTATION Transcriptional regulation of IFN-l genes in Hepatitis C virus-infected hepatocytes via IRF-3 IRF-7 NF- B complex Hai-Chon Lee *, Je-In Youn, Kyungwha Lee, Hwanyul Yong, Seung-Yong
POSTER PRESENTATION Transcriptional regulation of IFN-l genes in Hepatitis C virus-infected hepatocytes via IRF-3 IRF-7 NF- B complex Hai-Chon Lee *, Je-In Youn, Kyungwha Lee, Hwanyul Yong, Seung-Yong
F* techniques: FRAP, FLIP, FRET, FLIM,
 F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
Tracking sub-microscopic protein organisation at the plasma membrane of live cells using triple-colour superresolution
 Tracking sub-microscopic protein organisation at the plasma membrane of live cells using triple-colour superresolution microscopy Jacob Piehler Division of Biophysics, University of Osnabrück, Germany
Tracking sub-microscopic protein organisation at the plasma membrane of live cells using triple-colour superresolution microscopy Jacob Piehler Division of Biophysics, University of Osnabrück, Germany
Single-molecule specific mislocalization of red fluorescent proteins in live Escherichia coli
 University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2016 Single-molecule specific mislocalization of red fluorescent proteins
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2016 Single-molecule specific mislocalization of red fluorescent proteins
A guide to choosing fluorescent proteins
 A guide to choosing fluorescent proteins Nathan C Shaner 1,2, Paul A Steinbach 1,3 & Roger Y Tsien 1,3,4 The recent explosion in the diversity of available fluorescent proteins (FPs) 1 16 promises a wide
A guide to choosing fluorescent proteins Nathan C Shaner 1,2, Paul A Steinbach 1,3 & Roger Y Tsien 1,3,4 The recent explosion in the diversity of available fluorescent proteins (FPs) 1 16 promises a wide
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Accessorize Your Imaging. Ian Clements Invitrogen Corp
 Accessorize Your Imaging Ian Clements Invitrogen Corp Imaging Tools and Accessories Antifade Reagents Prolong Gold SlowFade Gold Image-iT FX Signal Enhancer FocalCheck Microscope Test Slides Fluorescent
Accessorize Your Imaging Ian Clements Invitrogen Corp Imaging Tools and Accessories Antifade Reagents Prolong Gold SlowFade Gold Image-iT FX Signal Enhancer FocalCheck Microscope Test Slides Fluorescent
Labeling cells with photoconvertible fluorescent proteins in zebrafish. Instructors: Debbie Yelon and Xin-Xin Zeng
 Labeling cells with photoconvertible fluorescent proteins in zebrafish Instructors: Debbie Yelon (dyelon@ucsd.edu) and Xin-Xin Zeng (xxzeng@ucsd.edu) Introduction: Photoconvertible (aka photoswitchable,
Labeling cells with photoconvertible fluorescent proteins in zebrafish Instructors: Debbie Yelon (dyelon@ucsd.edu) and Xin-Xin Zeng (xxzeng@ucsd.edu) Introduction: Photoconvertible (aka photoswitchable,
A Brief History of Light Microscopy And How It Transformed Biomedical Research
 A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
Imaging of BacMam Transfected U-2 OS Cells
 A p p l i c a t i o n N o t e Imaging of BacMam Transfected U-2 OS Cells Optimization of Transfection Conditions Using the Cytation 3 Multi- Mode Reader and Gen5 Data Analysis Software Paul Held Ph. D.
A p p l i c a t i o n N o t e Imaging of BacMam Transfected U-2 OS Cells Optimization of Transfection Conditions Using the Cytation 3 Multi- Mode Reader and Gen5 Data Analysis Software Paul Held Ph. D.
Fluorescent labeling of the nuclear envelope by localizing GFP on the inner nuclear membrane
 Supporting Information Fluorescent labeling of the nuclear envelope by localizing GFP on the inner nuclear membrane Toshiyuki Taniyama 1, Natsumi Tsuda 1, and Shinji Sueda 1,2,* 1 Department of Bioscience
Supporting Information Fluorescent labeling of the nuclear envelope by localizing GFP on the inner nuclear membrane Toshiyuki Taniyama 1, Natsumi Tsuda 1, and Shinji Sueda 1,2,* 1 Department of Bioscience
MEFs were treated with the indicated concentrations of LLOMe for three hours, washed
 Supplementary Materials and Methods Cell Fractionation MEFs were treated with the indicated concentrations of LLOMe for three hours, washed with ice-cold PBS, collected by centrifugation, and then homogenized
Supplementary Materials and Methods Cell Fractionation MEFs were treated with the indicated concentrations of LLOMe for three hours, washed with ice-cold PBS, collected by centrifugation, and then homogenized
SUPPLEMENTARY NOTE 2. Supplememtary Note 2, Wehr et al., Monitoring Regulated Protein-Protein Interactions Using Split-TEV 1
 SUPPLEMENTARY NOTE 2 A recombinase reporter system for permanent reporter activation We made use of the Cre-loxP recombinase system for a maximal amplification and complete kinetic uncoupling within single
SUPPLEMENTARY NOTE 2 A recombinase reporter system for permanent reporter activation We made use of the Cre-loxP recombinase system for a maximal amplification and complete kinetic uncoupling within single
Imaging facilities at WUR
 Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
The Nuclear Area Factor (NAF): a measure for cell apoptosis using microscopy and image analysis
 The Nuclear Area Factor (NAF): a measure for cell apoptosis using microscopy and image analysis Mark A. DeCoster Department of Biomedical Engineering and Institute for Micromanufacturing, Louisiana Tech
The Nuclear Area Factor (NAF): a measure for cell apoptosis using microscopy and image analysis Mark A. DeCoster Department of Biomedical Engineering and Institute for Micromanufacturing, Louisiana Tech
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Expression of uorescently tagged connexins: a novel approach to rescue function of oligomeric DsRed-tagged proteins 1
 FEBS 24888 FEBS Letters 498 (2001) 11^15 Expression of uorescently tagged connexins: a novel approach to rescue function of oligomeric DsRed-tagged proteins 1 Undine Lauf 2, Patricia Lopez 2, Matthias
FEBS 24888 FEBS Letters 498 (2001) 11^15 Expression of uorescently tagged connexins: a novel approach to rescue function of oligomeric DsRed-tagged proteins 1 Undine Lauf 2, Patricia Lopez 2, Matthias
Fluorescence Microscopy: A Biological Perspective
 Fluorescence Microscopy: A Biological Perspective From nanometre to metre: the scale of life Instrumentation and accessible scale limits the questions that can be addressed in biology Why are there limits?
Fluorescence Microscopy: A Biological Perspective From nanometre to metre: the scale of life Instrumentation and accessible scale limits the questions that can be addressed in biology Why are there limits?
Spectral Separation of Multifluorescence Labels with the LSM 510 META
 Microscopy from Carl Zeiss Spectral Separation of Multifluorescence Labels with the LSM 510 META Indians living in the South American rain forest can distinguish between almost 200 hues of green in their
Microscopy from Carl Zeiss Spectral Separation of Multifluorescence Labels with the LSM 510 META Indians living in the South American rain forest can distinguish between almost 200 hues of green in their
Confocal Microscopy & Imaging Technology. Yan Wu
 Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Resolution of Microscopes Visible light is nm Dry lens(0.5na), green(530nm light)=0.65µm=650nm for oil lens (1.4NA) UV light (300nm) = 0.13µm f
 Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
In vitro EGFP-CALI comprehensive analysis. Liang CAI. David Humphrey. Jessica Wilson. Ken Jacobson. Dept. of Cell and Developmental Biology
 In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
ab CytoPainter ER Staining Kit Red Fluorescence
 ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Fluorescence Microscopy
 Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Aoki et al.,
 JCB: SUPPLEMENTAL MATERIAL Aoki et al., http://www.jcb.org/cgi/content/full/jcb.200609017/dc1 Supplemental materials and methods Calculation of the raw number of translocated proteins First, the average
JCB: SUPPLEMENTAL MATERIAL Aoki et al., http://www.jcb.org/cgi/content/full/jcb.200609017/dc1 Supplemental materials and methods Calculation of the raw number of translocated proteins First, the average
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Craft et al., http://www.jcb.org/cgi/content/full/jcb.201409036/dc1 Figure S1. GFP -tubulin interacts with endogenous -tubulin. Ciliary
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Craft et al., http://www.jcb.org/cgi/content/full/jcb.201409036/dc1 Figure S1. GFP -tubulin interacts with endogenous -tubulin. Ciliary
Reviewer #1 (Remarks to the Author):
 Reviewer #1 (Remarks to the Author): This manuscript describes a novel application of dye-labeled DNA-conjugated AuNPs in visualizing intracellular transport within living cells. The most important result
Reviewer #1 (Remarks to the Author): This manuscript describes a novel application of dye-labeled DNA-conjugated AuNPs in visualizing intracellular transport within living cells. The most important result
Cre Stoplight with Living Colors is a faster, brighter
 Cre Stoplight with Living Colors is a faster, brighter reporter for Cre recombinase. Drago A Guggiana-Nilo 1, Anne Marie Quinn 2,Thomas E. Hughes 1 1 Department of Cell Biology and Neuroscience, Montana
Cre Stoplight with Living Colors is a faster, brighter reporter for Cre recombinase. Drago A Guggiana-Nilo 1, Anne Marie Quinn 2,Thomas E. Hughes 1 1 Department of Cell Biology and Neuroscience, Montana
Supplementary Figure S1. Np95 interacts with de novo methyltransferases Dnmt3a and 3b. (A) Co-immunoprecipitation of endogenous Dnmt3a2 (left and
 Supplementary Figure S1. Np95 interacts with de novo methyltransferases Dnmt3a and 3b. (A) Co-immunoprecipitation of endogenous Dnmt3a2 (left and right), Dnmt3b isoforms (left) and Dnmt1 (right) with GFP-Np95
Supplementary Figure S1. Np95 interacts with de novo methyltransferases Dnmt3a and 3b. (A) Co-immunoprecipitation of endogenous Dnmt3a2 (left and right), Dnmt3b isoforms (left) and Dnmt1 (right) with GFP-Np95
Self-labelling enzymes as universal tags for fluorescence microscopy, superresolution microscopy and electron microscopy
 Supplementary Materials Self-labelling enzymes as universal tags for fluorescence microscopy, superresolution microscopy and electron microscopy Viktoria Liss, Britta Barlag, Monika Nietschke and Michael
Supplementary Materials Self-labelling enzymes as universal tags for fluorescence microscopy, superresolution microscopy and electron microscopy Viktoria Liss, Britta Barlag, Monika Nietschke and Michael
Transcription factor dynamics in the nucleus
 Transcription factor dynamics in the nucleus March 21, 2018 Luca Giorgetti txnlecture2018@fmi.ch Transcription factors bind to enhancer and promoter regions Adapted from Streubel & Bracken EMBO J 2015
Transcription factor dynamics in the nucleus March 21, 2018 Luca Giorgetti txnlecture2018@fmi.ch Transcription factors bind to enhancer and promoter regions Adapted from Streubel & Bracken EMBO J 2015
Autophagy/Cytotoxicity Dual Staining Kit
 Autophagy/Cytotoxicity Dual Staining Kit Catalog Number KA1299 96 assays Version: 05 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
Autophagy/Cytotoxicity Dual Staining Kit Catalog Number KA1299 96 assays Version: 05 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
Illumatool ΤΜ Tunable Light System: A Non-Destructive Light Source For Molecular And Cellular Biology Applications. John Fox, Lightools Research.
 Illumatool ΤΜ Tunable Light System: A Non-Destructive Light Source For Molecular And Cellular Biology Applications. John Fox, Lightools Research. Fluorescent dyes and proteins are basic analytical tools
Illumatool ΤΜ Tunable Light System: A Non-Destructive Light Source For Molecular And Cellular Biology Applications. John Fox, Lightools Research. Fluorescent dyes and proteins are basic analytical tools
Imaging of Cells using fluorescents dyes. By: Josué A. Benjamín Rivera September 27, 2018
 Imaging of Cells using fluorescents dyes By: Josué A. Benjamín Rivera September 27, 2018 1 History Sir William Henry Perkin BRITISH CHEMIST In 1856, at the age of 18, William Henry Perkin set out with
Imaging of Cells using fluorescents dyes By: Josué A. Benjamín Rivera September 27, 2018 1 History Sir William Henry Perkin BRITISH CHEMIST In 1856, at the age of 18, William Henry Perkin set out with
Supplementary Figures
 Supplementary Figures a HindIII XbaI pcdna3.1/v5-his A_Nfluc-VVD 1-398 37-186 Nfluc Linker 1 VVD HindIII XhoI pcdna3.1/v5-his A_VVD-Nfluc 37-186 1-398 VVD Linker 2 Nfluc HindIII XbaI pcdna3.1/v5-his A_Cfluc-VVD
Supplementary Figures a HindIII XbaI pcdna3.1/v5-his A_Nfluc-VVD 1-398 37-186 Nfluc Linker 1 VVD HindIII XhoI pcdna3.1/v5-his A_VVD-Nfluc 37-186 1-398 VVD Linker 2 Nfluc HindIII XbaI pcdna3.1/v5-his A_Cfluc-VVD
Immunofluorescence images of different core histones and different histone exchange assay.
 Molecular Cell, Volume 51 Supplemental Information Enhanced Chromatin Dynamics by FACT Promotes Transcriptional Restart after UV-Induced DNA Damage Christoffel Dinant, Giannis Ampatziadis-Michailidis,
Molecular Cell, Volume 51 Supplemental Information Enhanced Chromatin Dynamics by FACT Promotes Transcriptional Restart after UV-Induced DNA Damage Christoffel Dinant, Giannis Ampatziadis-Michailidis,
Chapter One. Construction of a Fluorescent α5 Subunit. Elucidation of the unique contribution of the α5 subunit is complicated by several factors
 4 Chapter One Construction of a Fluorescent α5 Subunit The significance of the α5 containing nachr receptor (α5* receptor) has been a challenging question for researchers since its characterization by
4 Chapter One Construction of a Fluorescent α5 Subunit The significance of the α5 containing nachr receptor (α5* receptor) has been a challenging question for researchers since its characterization by
Sélection Internationale Ens Ulm 2012, Cell Biology
 Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Fluorescence Microscopy
 Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Automated Imaging Assay for Characterizing Ca 2+ Flux with R-GECO Biosensor
 A p p l i c a t i o n N o t e Automated Imaging Assay for Characterizing Ca 2+ Flux with R-GECO Biosensor Joe Clayton, Ph.D., and Peter Banks, Ph.D., BioTek Instruments, Inc., Winooski, VT USA Abstract
A p p l i c a t i o n N o t e Automated Imaging Assay for Characterizing Ca 2+ Flux with R-GECO Biosensor Joe Clayton, Ph.D., and Peter Banks, Ph.D., BioTek Instruments, Inc., Winooski, VT USA Abstract
Aminoacid change in chromophore. PIN3::PIN3-GFP GFP S65 (no change) (5.9) (Kneen et al., 1998)
 Supplemental Table. Table S1. Fluorophore characteristics of used fluorescent proteins, Related to Figure 2 and 3. Transgenic line Fluprescent marker Aminoacid change in chromophore pk(a) Ref. PIN3::PIN3-GFP
Supplemental Table. Table S1. Fluorophore characteristics of used fluorescent proteins, Related to Figure 2 and 3. Transgenic line Fluprescent marker Aminoacid change in chromophore pk(a) Ref. PIN3::PIN3-GFP
Supplemental Information. Dynamic Organization of Chromatin Domains. Revealed by Super-Resolution Live-Cell Imaging
 Molecular Cell, Volume 67 Supplemental Information Dynamic Organization of Chromatin Domains Revealed by Super-Resolution Live-Cell Imaging Tadasu Nozaki, Ryosuke Imai, Mai Tanbo, Ryosuke Nagashima, Sachiko
Molecular Cell, Volume 67 Supplemental Information Dynamic Organization of Chromatin Domains Revealed by Super-Resolution Live-Cell Imaging Tadasu Nozaki, Ryosuke Imai, Mai Tanbo, Ryosuke Nagashima, Sachiko
CytoPainter Golgi Staining Kit Green Fluorescence
 ab139483 CytoPainter Golgi Staining Kit Green Fluorescence Instructions for Use Designed for the detection of Golgi bodies by microscopy This product is for research use only and is not intended for diagnostic
ab139483 CytoPainter Golgi Staining Kit Green Fluorescence Instructions for Use Designed for the detection of Golgi bodies by microscopy This product is for research use only and is not intended for diagnostic
Lab 5: Optical trapping and single molecule fluorescence
 Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
CENTRAL FACILITY BIOOPTICS MFPL
 CENTRAL FACILITY BIOOPTICS MFPL LIVE IMAGING CONSIDERATIONS Why Live Imaging? You can (somehow) believe what you see no fixation artefacts Follow the order of events in real-time Monitor dynamics: kinetics,
CENTRAL FACILITY BIOOPTICS MFPL LIVE IMAGING CONSIDERATIONS Why Live Imaging? You can (somehow) believe what you see no fixation artefacts Follow the order of events in real-time Monitor dynamics: kinetics,
Visualizing Cells Molecular Biology of the Cell - Chapter 9
 Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Workshop advanced light microscopy
 Workshop advanced light microscopy Multi-mode confocal laser scanning microscope Jan Willem Borst Laboratory of Biochemistry Biomolecular Networks www.bic.wur.nl MicroSpectroscopy Centre Wageningen Microspectroscopy
Workshop advanced light microscopy Multi-mode confocal laser scanning microscope Jan Willem Borst Laboratory of Biochemistry Biomolecular Networks www.bic.wur.nl MicroSpectroscopy Centre Wageningen Microspectroscopy
ab CytoPainter ER Staining Kit Red Fluorescence
 ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material THE JOURNAL OF CELL BIOLOGY Yeung et al., http://www.jcb.org/cgi/content/full/jcb.200903020/dc1 Figure S1. Assessment of the surface charge of maturing phagosomes. (A C and F H) RAW
Supplemental Material THE JOURNAL OF CELL BIOLOGY Yeung et al., http://www.jcb.org/cgi/content/full/jcb.200903020/dc1 Figure S1. Assessment of the surface charge of maturing phagosomes. (A C and F H) RAW
Imaging of endocrine organs
 Imaging of endocrine organs Helen Christian Department of Physiology, Anatomy & Genetics St Anne s College, University of Oxford Diabetesforum, Stockholm 2017 Islets of Langerhan Pituitary gland Renin
Imaging of endocrine organs Helen Christian Department of Physiology, Anatomy & Genetics St Anne s College, University of Oxford Diabetesforum, Stockholm 2017 Islets of Langerhan Pituitary gland Renin
over time using live cell microscopy. The time post infection is indicated in the lower left corner.
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Supplementary Movie 1 Description: Fusion of NBs. BSR cells were infected
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Supplementary Movie 1 Description: Fusion of NBs. BSR cells were infected
Monomeric fluorescent timers that change color from blue to red report on cellular trafficking
 Monomeric fluorescent timers that change color from blue to red report on cellular trafficking Fedor V. Subach, Oksana M. Subach, Illia S. Gundorov, Kateryna S. Morozova, Kiryl D. Piatkevich, Ana Maria
Monomeric fluorescent timers that change color from blue to red report on cellular trafficking Fedor V. Subach, Oksana M. Subach, Illia S. Gundorov, Kateryna S. Morozova, Kiryl D. Piatkevich, Ana Maria
Non-coding Function & Variation, MPRAs. Mike White Bio5488 3/5/18
 Non-coding Function & Variation, MPRAs Mike White Bio5488 3/5/18 Outline MONDAY Non-coding function and variation The barcode Basic versions of MRPA technology WEDNESDAY More varieties of MRPAs Some key
Non-coding Function & Variation, MPRAs Mike White Bio5488 3/5/18 Outline MONDAY Non-coding function and variation The barcode Basic versions of MRPA technology WEDNESDAY More varieties of MRPAs Some key
3D Transfection System
 3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
Supplementary Information
 Supplementary Information STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA Iddo Heller, Gerrit Sitters, Onno D. Broekmans, Géraldine Farge, Carolin Menges,
Supplementary Information STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA Iddo Heller, Gerrit Sitters, Onno D. Broekmans, Géraldine Farge, Carolin Menges,
Chapter 4. the biological community to assay for protein-protein interactions. FRET describes the
 31 Chapter 4 Determination of nachr stoichiometry using Normalized Försters Resonance Energy Transfer (NFRET) Försters resonance energy transfer (FRET) has become a technique widely used in the biological
31 Chapter 4 Determination of nachr stoichiometry using Normalized Försters Resonance Energy Transfer (NFRET) Försters resonance energy transfer (FRET) has become a technique widely used in the biological
SUPPLEMENTARY INFORMATION
 DOI: 1.18/ncb46 a Damage Fracture s s 7 5 16 1 9 26 5 µm 5 µm b Position of photo-damage site in cells 15 Counts 1 5-14 -12-1 -8-6 -4-2 Distance from damage site to tip (µm) Supplementary Figure 1 Effect
DOI: 1.18/ncb46 a Damage Fracture s s 7 5 16 1 9 26 5 µm 5 µm b Position of photo-damage site in cells 15 Counts 1 5-14 -12-1 -8-6 -4-2 Distance from damage site to tip (µm) Supplementary Figure 1 Effect
Gene disruption and construction of C-terminally tagged strains
 Supplementary information Supplementary Methods Gene disruption and construction of C-terminally tagged strains A PCR-based gene targeting method (Bähler et al., 1998; Sato et al., 2005) was used for constructing
Supplementary information Supplementary Methods Gene disruption and construction of C-terminally tagged strains A PCR-based gene targeting method (Bähler et al., 1998; Sato et al., 2005) was used for constructing
TECHNICAL BULLETIN. In Vitro Bacterial Split Fluorescent Protein Fold n Glow Solubility Assay Kits
 In Vitro Bacterial Split Fluorescent Protein Fold n Glow Solubility Assay Kits Catalog Numbers APPA001 In Vitro Bacterial Split GFP "Fold 'n' Glow" Solubility Assay Kit (Green) APPA008 In Vitro Bacterial
In Vitro Bacterial Split Fluorescent Protein Fold n Glow Solubility Assay Kits Catalog Numbers APPA001 In Vitro Bacterial Split GFP "Fold 'n' Glow" Solubility Assay Kit (Green) APPA008 In Vitro Bacterial
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Contents. SCHOOL of FLUORESCENCE. For more information, go to lifetechnologies.com/imagingbasics
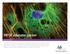 MPSF educator packet This packet contains illustrations and figures from the Molecular Probes School of Fluorescence website. They illustrate concepts from the basic physical properties that underlie fluorescence
MPSF educator packet This packet contains illustrations and figures from the Molecular Probes School of Fluorescence website. They illustrate concepts from the basic physical properties that underlie fluorescence
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
