Intermediate filaments (IF)1 are major cytoskeletal
|
|
|
- Dorcas Young
- 6 years ago
- Views:
Transcription
1 Motile Properties of Vimentin Intermediate Filament Networks in Living Cells Miri Yoon, Robert D. Moir, Veena Prahlad, and Robert D. Goldman Northwestern University Medical School, Department of Cell and Molecular Biology, Chicago, Illinois Abstract. The motile properties of intermediate filament (IF) networks have been studied in living cells expressing vimentin tagged with green fluorescent protein (GFP-vimentin). In interphase and mitotic cells, GFPvimentin is incorporated into the endogenous IF network, and accurately reports the behavior of IF. Timelapse observations of interphase arrays of vimentin fibrils demonstrate that they are constantly changing their configurations in the absence of alterations in cell shape. Intersecting points of vimentin fibrils, or foci, frequently move towards or away from each other, indicating that the fibrils can lengthen or shorten. Fluorescence recovery after photobleaching shows that bleach zones across fibrils rapidly recover their fluorescence. During this recovery, bleached zones frequently move, indicating translocation of fibrils. Intriguingly, neighboring fibrils within a cell can exhibit different rates and directions of movement, and they often appear to extend or elongate into the peripheral regions of the cytoplasm. In these same regions, short filamentous structures are also seen actively translocating. All of these motile properties require energy, and the majority appear to be mediated by interactions of IF with microtubules and microfilaments. Key words: intermediate filaments vimentin microtubules microfilaments green fluorescent protein Intermediate filaments (IF)1 are major cytoskeletal components of mammalian cells that appear to be relatively stable structures because of their insolubility in vitro and the lack of biochemical evidence for the existence of significant pools of soluble subunits in vivo (Steinert et al., 1976; Zackroff and Goldman, 1979; Soellner et al., 1985). However, there is increasing evidence that intermediate filaments are dynamic structures in vivo. For example, they form juxtanuclear aggregates in response to microinjection of IF antibodies and heat shock (Klymkowsky, 1981; Collier et al., 1993). In mitosis, partial or complete disassembly of IF networks takes place, followed by their reassembly in daughter cells (Franke et al., 1982; Rosevear et al., 1990). Furthermore, the state of IF polymerization is regulated by subunit exchange in vivo as suggested by the findings that microinjected vimentin and keratin, as well as newly synthesized IF proteins, become incorporated into Address all correspondence to Robert D. Goldman, Northwestern University Medical School, Department of Cell and Molecular Biology, 303 E. Chicago Ave., Chicago, IL Tel.: Fax: r-goldman@nwu.edu 1. Abbreviations used in this paper: GFP, green fluorescent protein; IF, intermediate filaments; MT, microtubules; MF, microfilaments; FRAP, fluorescence recovery after photobleaching; t 1/2, recovery half-time. preexisting IF networks (Vikstrom et al., 1989; Albers and Fuch, 1989; Ngai et al., 1990; Miller et al., 1993). The most direct evidence supporting an equilibrium between unpolymerized IF subunits and polymerized IF in vivo has been obtained from fluorescence recovery after photobleaching (FRAP) analyses (Vikstrom et al., 1992; Okabe et al., 1993). Interactions of IF with IF-associated proteins (IFAPs), microtubules (MT), and microfilaments (MF) are thought to regulate IF organization in vivo (Chou et al., 1997). Structural evidence for IF MT interactions comes from the observation of close parallel arrays of IF and MT, as well as the reorganization of IF to form perinuclear caps in response to MT depolymerization (Goldman and Knipe, 1973). Furthermore, MAP-2 (Hirokawa et al., 1988) and the IFAP plectin (Svitkina et al., 1996) have been shown to form cross-bridges between IF and MT. Evidence supporting IF MF interactions comes from ultrastructural observations demonstrating close associations between IF and MF (Schliwa and van Blerkom, 1981), and from the finding that colcemid-induced movement of vimentin IF into perinuclear caps is inhibited by cytochalasin D (Hollenbeck et al., 1989). Candidates for cross-bridging elements between IF and MF include plectin and dystonin/ BPAG1n (Brown et al., 1995; McLean et al., 1996; Yang et al., 1996). Very little is known about the motile properties of IF due primarily to the difficulties inherent in visualizing IF The Rockefeller University Press, /98/10/147/11 $2.00 The Journal of Cell Biology, Volume 143, Number 1, October 5,
2 in vivo. Recently, many of these difficulties have been alleviated by expression of proteins tagged with the green fluorescent protein (GFP; Cubitt et al., 1995). Direct observations of GFP-tagged cytoskeletal proteins in vivo have provided insights into the dynamic properties of MT, MAP-4, actin, and IF (Olson et al., 1995; Ludin and Matus, 1998; Fischer et al., 1998; Ho et al., 1998). In this study we describe in detail the motile properties of vimentin IF using GFP fused with vimentin (GFP-vimentin). After transfection with GFP-vimentin, time-lapse and FRAP analyses show that IF networks constantly change their configurations in vivo. GFP-vimentin fibrils are motile; they can translocate, alter their shapes, and apparently extend and shorten over relatively short time intervals. Time-lapse imaging of GFP-vimentin at the cell periphery reveals unexpected movement of short filamentous structures (termed squiggles). The effects of nocodazole and cytochalasin B indicate that many, but not all of these motile properties of IF are dependent on MT and/or MF. Materials and Methods Cloning of GFP-vimentin cdna Constructs A GFP-vimentin construct was made by fusing GFP to the NH 2 -terminus of human vimentin cdna. A BamHI BamHI fragment containing the human vimentin cdna sequence, with or without the human c-myc tag (Chou et al., 1996) was subcloned into the BamHI site of pegfp-c1 (CLONTECH Laboratories Inc., Palo Alto, CA). The results obtained in cells transfected with GFP-vimentin with or without the c-myc tag were identical. Cell Cultures and Transient Transfection Baby hamster kidney (BHK-21) fibroblasts were grown in DME supplemented with 10% tryptose phosphate broth (Difco Laboratories Inc., Detroit, MI), 10% calf serum, and antibiotics (100 U/ml penicillin and streptomycin). SW13 vim cells (a gift of Dr. Robert Evans, University of Colorado) were grown in DME with 10% FCS and antibiotics. Bovine pulmonary arterial endothelial cells (CPAE; American Type Culture Collection, Rockville, MD) were grown in DME with 2 mm glutamine, 10% FCS, and antibiotics. HeLa and PtK2 cells were grown in MEM with 10% FCS, 0.1 mm nonessential amino acid solution (GIBCO-BRL, Gaithersburg, MD), and antibiotics. For transfection, Lipofectamine (GIBCO-BRL) was used according to the manufacturer s protocol. Cells grown on coverslips were incubated with a mixture of 1 g of CsCl-purified DNA and 6 l of Lipofectamine in serum-free medium for 3 h, after which the mixture was replaced with standard culture medium. Preparation of IF-enriched Cytoskeletons and Immunoprecipitation IF-enriched cytoskeletal preparations were made from confluent BHK-21 cell cultures (Zackroff and Goldman, 1979), and the final pellet was solubilized and sonicated in 10 mm Tris-HCl, ph 6.8, 5 mm EDTA, 2% SDS, 10% glycerol, and Complete protease inhibitors (Boehringer Mannheim Corp., Indianapolis, IN). The resulting solution was diluted 1:20 in 20 mm Tris-HCl, ph 7.5, 150 mm NaCl, 1% NP-40, 5 mm EDTA, and Complete protease inhibitors, and was used for immunoprecipitation experiments. Immunoprecipitation of GFP-vimentin was carried out with Protein A-sepharose (Sigma Chemical Co., St. Louis, MO) and 9E10, a mouse monoclonal anti-human c-myc antibody (Evan et al., 1985; American Type Culture Collection) as described elsewhere (Chou et al., 1990). The immunoprecipitates were separated by SDS-PAGE (Laemmli, 1970). Immunoblotting was carried out according to Towbin et al. (1979). The primary antibodies used were 9E10 and a mouse monoclonal anti-vimentin (Sigma Chemical Co.). The secondary antibody used was peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Indirect Immunofluorescence Cells grown on coverslips were fixed and processed for indirect immunofluorescence as described by Yang et al. (1985). The primary antibodies used were 9E10, a rabbit polyclonal anti-bhk IF (Yang et al., 1985), a rabbit polyclonal anti-bovine tongue keratin antibody (Jones et al., 1988) and a mouse monoclonal anti- -tubulin (Amersham Life Science, Inc., Arlington Heights, IL). The secondary antibodies used were fluoresceinconjugated goat anti mouse IgG and Lissamine rhodamine (LRSC)-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc.). Microscopy For live cell studies, transfected cells were trypsinized 48 h after transfection, and were plated onto coverslips to achieve 70% confluence in standard culture medium containing 10 mm Hepes, ph 7.0. The coverslips were placed on small glass feet (to prevent compression) on slides, sealed with a mixture of Vaseline, beeswax, and lanolin (1:1:1), and maintained at 37 C with an air stream stage incubator (Model ASI 400; NEVTEK, Burnsville, VA). In some cases, these coverslips were treated with nocodazole (Sigma Chemical Co.) at final concentrations of either 100 or 600 nm. In other cases, cytochalasin B (Sigma Chemical Co.) was added at final concentrations of either 2 or 20 M. For metabolic inhibition studies, final concentrations of both 50 mm 2-deoxy-d-glucose (Sigma Chemical Co.) and 0.05% sodium azide (Sigma Chemical Co.) were added. For cellcycle studies, chromosomes were stained with 2 g/ml of Hoechst (Molecular Probes Inc., Eugene, OR). Time-lapse observations were made with an LSM 410 confocal microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a 100, 1.4 NA oil immersion objective. Phase-contrast images of cells were taken before and after time-lapse observations to ensure that no significant change in cell shape or position occurred during the periods of observation. Digitized images were collected every 30 s for min using a fluorescein filter set (excitation at 488 nm and emission at nm). To test the stability of the focal plane in the confocal microscope, we made time-lapse observations of MT patterns for 30 min in fixed BHK cells after staining with anti- -tubulin. No obvious change in focus was detected during this time interval. This minimized problems due to focal plane drift during the periods of observation. In some cases, indirect immunofluorescence observations were made using a Zeiss Axiophot with a CCD camera (Photometrics, Tucson, AZ) controlled by Metamorph imaging software (Universal Imaging Corp., West Chester, PA). Fluorescence Recovery After Photobleaching (FRAP) FRAP was carried out with the LSM 410 confocal microscope (Carl Zeiss, Inc., Thornwood, NY). Phase-contrast images of cells were taken before and immediately after FRAP to ensure that there were no significant changes in cell shape or position. Bar-shaped regions were bleached using the line-scan function at 488 nm (100% power, 1% attenuation), and recovery of fluorescence was monitored (15 30% power, 10% attenuation) using the time-series function at 2-min intervals for up to 1 h. Image Analyses Position, length, and intensity measurements were made on digitized confocal images using Metamorph image analysis software. Pixel values were converted to distance using confocal calibration bars. Analyses of the dynamic properties of GFP-vimentin fibrils were restricted to cells that showed no obvious shape changes for min. If the average displacement of a cell s margins was more than 2 m in 40 min (0.05 m/min) as indicated by phase contrast, it was excluded from the analyses. Rates of translocation of vimentin fibrils were determined by monitoring distance vs. time. If vimentin fibrils or foci moved at least 0.25 m in 5 min (0.05 m/min), the data were used to calculate the rates of movements. FRAP half-times (t 1/2 ) were calculated by monitoring the gray-scale pixel values (intensity measurements) across the bleached vimentin fibrils using the line-scan function of Metamorph at each time point after adjustment for sample fading during time-lapse observations (see Vikstrom et al., 1992). All data are reported as mean SD. The Journal of Cell Biology, Volume 143,
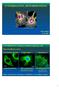
3 Results Figure 1. GFP-vimentin IF networks in interphase cells 72 h after transfection. (A and B) Phase-contrast (A) and fluorescence (B) images of a live BHK cell show that GFP-vimentin IF networks extend from the perinuclear region to the cell periphery. (C and D) In PtK2 cells that contain vimentin and keratin IF networks, GFP-vimentin is incorporated into the vimentin IF network, as indicated by fixation and double indirect immunofluorescence. GFP-vimentin-myc (C) was visualized with monoclonal antihuman c-myc antibody and keratin (D) with polyclonal antibovine tongue keratin antibody. (E and F) Phase-contrast (E) and GFP (F) images of a live BHK cell after treatment with 600 nm nocodazole for 5 h. The majority of the vimentin IF network is reorganized into a perinuclear cap. Bar, 5 m. Characterization of the GFP-vimentin Fusion Protein In Vivo and In Vitro GFP-vimentin was expressed in 30% of the BHK-21 cells 72 h after transfection. The majority of these cells displayed typical cytoplasmic IF networks (Fig. 1, A and B), indistinguishable from those seen by immunofluorescence (Yang et al., 1985). GFP-vimentin colocalized with endogenous vimentin as demonstrated by double-label immunofluorescence (see Fig. 6, B, and E F). GFP-vimentin also formed typical IF networks in CPAE endothelial cells (see Fig. 5). In HeLa (data not shown) and PtK2 cells that have two IF networks, GFP-vimentin was incorporated only into the vimentin and not the keratin IF network (Fig. 1, C and D). GFP-vimentin does not appear to alter the properties of interphase IF networks as indicated by observations such as their reorganization into a perinuclear cap after the disassembly of MT (Fig. 1, E and F; see Yang et al., 1992). To ensure further that transfected cells accurately display the properties of IF, the cell cycle dependent behavior of GFP-vimentin was studied in live cells. In mitotic BHK-21 cells, the fluorescent IF networks disassembled into nonfilamentous aggregates (Fig. 2, A, B, D, and E), and during cytokinesis reassembled into a juxtanuclear cap in each daughter cell (Fig. 2, C and F). These observations are very similar to those seen by immunofluorescence (Rosevear et al., 1990). In PtK2 cells, GFP-vimentin IF aggregated around the mitotic spindle (Fig. 2, G, H, J, and K), and were distributed to daughter cells in cytokinesis coincident with a loss of fluorescence in the midbody region (Fig. 2, I and L). This was also identical to the results obtained by immunofluorescence (Aubin et al., 1980). Therefore, GFP-vimentin follows the normal progression of changes of IF during cell division and acts as a faithful reporter of IF in live cells. We have also attempted to establish GFP-vimentin IF networks in cells lacking vimentin. To this end, SW13 vim cells were transfected with GFP-vimentin. Under these conditions, 4 5% of the cells expressed GFP-vimentin. However, only 1% of these displayed typical IF patterns, while the remainder displayed only aggregates of vimentin (data not shown; see Ho et al., 1998). This low level of expression of typical IF networks may be related to the leaky expression of vimentin that we detected in 1% of SW13 vim cells. Furthermore, when these vim cells were cotransfected with wild-type vimentin (Chou et al., 1996) and GFP-vimentin, 20% of the cells displayed typical IF networks (data not shown). These results indicate that GFP-vimentin is capable of assembling into IF only in the presence of a wild-type background. GFP-vimentin with the c-myc tag was tested at the biochemical level for its ability to incorporate into endogenous IF networks in BHK-21 cells. 72 h after transfection, IF-enriched cytoskeletons were prepared from cultures with 30% transfected cells. SDS-PAGE analyses indicated the presence of vimentin, other IF-associated proteins, and a minor 82-kD band corresponding to the molecular weight of GFP-vimentin (Fig. 3 A). Immunoblot analyses of these preparations showed that the 82-kD band reacted with c-myc antibody (Fig. 3 B). These cytoskeletal preparations were also solubilized, immunoprecipitated with c-myc antibody, and analyzed by SDS- PAGE and immunoblotting. The results showed a single band of 82 kd recognized by c-myc antibody (Fig. 3 C), and both the 82-kD fusion protein and 55-kD untagged vimentin with vimentin antibody (Fig. 3 D). These results Yoon et al. Motility of Intermediate Filaments 149
4 Figure 2. Dynamic properties of GFPvimentin in live cells during mitosis. (A F) Mitotic BHK-21 cells. BHK cell in prometaphase showing chromosomes stained with Hoechst (A) and GFP-vimentin nonfilamentous aggregates (D). Phasecontrast (B) and fluorescence (E) images in anaphase. Phase-contrast (C) and GFP (F) images show reassembly into juxtanuclear caps in two daughter cells. (G L). Phase-contrast (G, H, and I) and fluorescent (J, K, and L) images of PtK2 cells showing the cage-like structure of vimentin fibrils during pro-metaphase (G and J), telophase (H and K) and in late cytokinesis (I and L). Note the loss of fluorescence in the region of the midbody in L. L is the same cell as K, taken 10 min later. Bar, 5 m. provide further support that GFP-vimentin is incorporated into the endogenous IF network. Figure 3. Analysis of an IFenriched cytoskeletal preparation of BHK-21 cells 72 h after transfection with GFP-vimentinmyc. SDS-PAGE analysis (A) reveals the presence of vimentin/desmin (*) and other proteins that coisolate with IF such as the nuclear lamins (NL) as well as 82-kD GFP-vimentinmyc, which is detected by immunoblotting with c-myc antibody (B). When these preparations were solubilized and immunoprecipitated with the c-myc antibody, GFP-vimentin-myc as well as endogenous vimentin were found in the immunoprecipitates. C is an immunoblot of the immunoprecipitate using the c-myc antibody showing a single 82-kD band; and D shows a blot of the same preparation using a monoclonal antibody directed against vimentin (V9; Sigma Chemical Co.) showing both the 82-kD GFP-vimentin and 55-kD untagged vimentin. Time-lapse Observations of GFP-vimentin IF Networks in Interphase Cells Time-lapse observations of GFP-vimentin IF networks were made in well-spread BHK-21 cells 72 h after transfection. Details of changes in the morphological features of GFP-vimentin IF networks were determined by timelapse recording at 30-s intervals for min. Analyses were restricted to cells that showed no obvious shape changes, as determined from phase-contrast images taken before and at the end of the time-lapse observations (see Materials and Methods). This procedure ensured that we were monitoring intrinsic changes in IF networks and not passive displacements due to significant changes in cell shape. The fluorescent IF networks were characterized by extensive arrays of fibrils. In some cases, fibrils appeared to be interconnected at diffusely fluorescent focal points (foci). The majority of the vimentin fibrils and foci displayed movements during the periods of observation. These foci were useful indicators of motility as they frequently moved towards or away from each other (Fig. 4, A C). The average rate of translocation of the foci and therefore changes in the length of the interconnecting fibrils was m/min (n 21). In no case did we observe a change in the direction of the movement of foci. However, this might be due to the limited time intervals of observation. Other types of movements frequently seen included significant shape changes in fibrils, as indicated by bending and/or straightening (Fig. 4, D F). Fibrils also appeared or disappeared in the focal plane (Fig. 4, G H). In addition, numerous fibrils appeared to extend into regions contain- The Journal of Cell Biology, Volume 143,
5 Figure 4. Time-lapse observations of GFP-vimentin IF networks in live BHK-21 cells. (A C) GFP-vimentin fibrils often interconnect bright foci (arrows), and in this case, the foci move towards each other while the fibrils interconnecting these foci appear to shorten. (D F) Vimentin fibrils can change their shapes, as indicated by bending (arrows). (G H) GFP-vimentin fibrils often appear in the focal plane over relatively short time intervals (arrows). Lapsed time (min:sec) is indicated at lower left. Bar, 5 m. ing a few IF at the cell periphery. This was especially evident in the extremely thin edges of CPAE cells. The average rate of extension at the cell periphery was m/ min with a range of m/min (n 30; Fig. 5, A I). FRAP Analyses In our earlier FRAP studies, after microinjecting rhodamine-labeled vimentin, we showed that there is an equilibrium between unpolymerized vimentin IF subunits and Figure 5. Time-lapse observations of GFP-vimentin fibrils in a live CPAE cell. Phase-contrast (A) and GFP images (B I) indicate that GFP-vimentin fibrils extend into a region lacking IF at the edge of a cell. Intervals between frames are 1 min. Bar, 5 m. Yoon et al. Motility of Intermediate Filaments 151
6 Figure 6. Continuity of vimentin fibrils across bleach zones following photobleaching. A GFP-vimentin-myc expressing BHK-21 cell was fixed at 1 min after photobleaching, and was processed for indirect immunofluorescence. The continuous vimentin-staining pattern across the bleach zones indicates that photobleaching does not damage vimentin fibrils. (A C) This series depicts a live cell in phase-contrast (A) and GFP before (B) and immediately after (C) photobleaching. (D F) Phase-contrast image (D). GFP-vimentin (E) is visualized with c-myc antibody and vimentin (F) with a polyclonal BHK IF antibody (F) in the same cell after fixation and processing for double indirect immunofluorescence. Bar, 10 m. polymerized vimentin IF in vivo. The main drawback of our previous study was our inability to estimate the average recovery half-time (t 1/2 ) due to relatively rapid background photobleaching (Vikstrom et al., 1992). A major advantage of using GFP fusion proteins is that fluorescence intensity does not decrease significantly during image acquisition over relatively long periods (Cubitt et al., 1995). Since our observations suggested that many fibrils were motile (Figs. 4, 5), FRAP analyses also provided another means of monitoring IF translocation during recovery, especially in regions of the cytoplasm containing parallel arrays of fibrils without a significant number of interconnecting foci. To ensure that photobleaching did not damage vimentin IF in vivo, BHK cells expressing GFP-vimentin-myc were fixed 1 min after bleaching, and were processed for double indirect immunofluorescence (Fig. 6). The continuous vimentin-staining patterns across the bleach zones indicated Figure 7. Time-lapse observations of FRAP in GFP-vimentin fibrils in a BHK-21 cell. (A D) The bleached GFP-vimentin fibrils completely recover their fluorescence within 20 min. (E H) Frequently the bleach zones on individual fibrils move at different rates during fluorescence recovery, as indicated by the transformation of the straight bleach zone into a wavy line. Lapsed time (min:sec) is indicated at the lower left of each image. Bar, 10 m. The Journal of Cell Biology, Volume 143,
7 that photobleaching did not disrupt the integrity of the vimentin fibrils. Time-lapse observations were made at 2-min intervals for 60 min after photobleaching. In some cytoplasmic regions, bleach zones were relatively stationary during recovery (Fig. 7, A D). FRAP analyses of such stationary fibrils indicated that the fluorescence intensities along bleached vimentin fibrils increased with time, and that no preferential recovery from either side could be detected (Fig. 8). The average time for the bleach zones to completely recover their fluorescence was min with an average t 1/2 of 5 3 min (n 25). In many instances bleach zones across fibrils moved during recovery. Bleach zones on closely spaced parallel fibrils either moved towards the nucleus or in the opposite direction towards the cell surface (Fig. 7, E H). This suggests that individual neighboring fibrils can translocate independently. The average rate of translocation of fibrils as determined from these motile bleach zones was m/min (n 23). Figure 8. Fluorescence intensity measurements along a photobleached GFP-vimentin fibril. Gray-scale pixel values were determined along a bleached vimentin fibril every 2 min with the Metamorph image analysis program. In this case, complete recovery took place in 14 min with a recovery half-time (t 1/2 ) of 6 min. Motile Properties of Short Vimentin Fibrils In addition to the typical networks of interconnecting fibrils seen in transfected cells, short filamentous structures termed vimentin squiggles were frequently visible. These were most apparent in the thin peripheral regions of BHK-21 cells (Fig. 9, A F). Vimentin squiggles were also seen in fixed untransfected BHK-21 and PtK2 cells (data not shown). Time-lapse observations of these squiggles showed that the majority were motile. Of 269 squiggles monitored for 10 min or more, 237 moved a distance of at least 0.5 m (237/269 88%). The majority of the motile squiggles (231/237 97%) moved towards the cell periphery. In some cases, squiggles reached the edge of the cell where they altered their shapes to reorient and move parallel to the cell surface (Fig. 9, D F). Squiggles translocated at speeds ranging from 1.3 to 11.4 m/min (n 32), with an average rate of m/min in BHK-21 cells. It should be noted that squiggles frequently underwent shape changes, even when they were not translocating. Nocodazole Treatment Affects the Motile Properties of IF Low concentrations of nocodazole (e.g., 100 nm) are known to interfere with MT dynamics without significantly affecting the number or distribution of MT (Liao et al., 1995; Vasquez et al., 1997). The overall distributions of MT and IF in BHK-21 cells treated with 100 nm nocodazole for min were similar to those of untreated cells as determined by fixation and double indirect immunofluorescence (data not shown). To determine whether the motile properties of GFP-vimentin fibrils in vivo were dependent on MT dynamics, we carried out time-lapse observations and FRAP analyses after adding 100 nm nocodazole for 30 min. Vimentin-rich foci continued to move Figure 9. Vimentin squiggles in live BHK- 21 cells. (A C) Phase-contrast (A) and fluorescence (B and C) images showing that squiggles are present in the peripheral region of the same cell. (D F) Timelapse observations demonstrating that the majority of vimentin squiggles are motile. The squiggle indicated by the top arrow shows a bending movement and a change of direction at the cell surface. Time intervals between frames are 30 s. Bar, 5 m. Yoon et al. Motility of Intermediate Filaments 153
8 Figure 10. GFP-vimentin in live BHK-21 cells treated with nocodazole, cytochalasin B, and metabolic inhibitors. (A) Cell treated with 600 nm nocodazole for 5 h. Note that the majority of vimentin fibrils are reorganized into a perinuclear cap, but that some fibrils remain associated with the cell surface. (B and C) Nocodazole-insensitive fibrils seen in A at higher magnification to show that shape changes continue in the absence of microtubules. (D) Phase-contrast image of the edge of a cell 30 min after the addition of 20 M cytochalasin B. Cell margins have retracted and the overall morphology becomes arborized. (E and F) GFP-vimentin squiggles are present in the same region of this cell. Two squiggles (arrows) showing translocations in the 30-s interval between these two images. (G and H) Motility of GFP-vimentin fibrils is completely arrested 15 min after adding 50 mm 2-deoxy-d-glucose and 0.05% sodium azide. This is most obvious when observing squiggles at the edge of a cell (see arrows). Lapsed time (min:sec) is indicated at lower left of figures. Bar, 5 m. relative to each other, and the fibrils interconnecting them appeared to shorten or lengthen at an average rate of m/min (n 16), representing a decrease of 58% compared with control cells. Fibrils also continued to change their shapes under these experimental conditions. FRAP analyses indicated that fibrils recovered their fluorescence more slowly at a t 1/2 of 8 5 min (n 16). During recovery, some bleach zones moved at an average rate of m/min (n 22), a 43% decrease relative to control cells. At the cell periphery, 93% (296/318) of the squiggles translocated at an average rate of m/ min (n 16), ranging from 1.5 to 9.9 m/min, which was similar to controls. We also studied fibril extension at the edges of endothelial cells in this concentration of nocodazole and found the average rate was m/min (n 20), which was also similar to controls. When BHK cells were treated with 600 nm nocodazole for 5 h, MT depolymerized (data not shown), and the majority of vimentin IF reorganized into perinuclear caps (Fig. 1, E F). Under these conditions, some vimentin fibrils remained extended towards the cell periphery, and squiggles could be found near the cell surfaces; however, it was difficult to locate foci (Fig. 10 A; see Yang et al., 1992). Time-lapse observations showed that the extended fibrils continued to change their shapes (Fig. 10, B and C), and FRAP analyses showed that they recovered their fluorescence more slowly at a t 1/2 of 8 3 min (n 12). During recovery, some bleach zones moved at a rate of m/min (n 7), representing a 60% decrease compared with controls. The majority of vimentin squiggles (381/400 95%) did not move during the periods of observation. However, 5% of the squiggles continued to move at a rate of m/min (n 19; range m/min), which was similar to controls. Inhibition of Actin Function Affects IF Motility Cytochalasin B was used to determine whether vimentin motility was dependent on MF. It has been shown that 2 M cytochalasin B inhibits actin-related functions such as membrane ruffling and cell locomotion without significantly affecting cell shape or the overall distribution of IF, MF, or MT (Goldman and Knipe, 1973). After treatment with 2 M cytochalasin B for 30 min in GFP-vimentin transfected BHK-21 cells, foci moved at an average rate of m/min (n 12), a 58% decrease relative to controls. FRAP analyses demonstrated a complete, although somewhat slower recovery with a t 1/2 of 10 5 min (n 17). During recovery, some bleach zones moved at an average rate of m/min (n 13), a 38% decrease compared with controls, while the majority (134/ %) of the squiggles continued to translocate at a The Journal of Cell Biology, Volume 143,
9 rate similar to controls ( m/min, n 19; range m/min). When transfected cells were exposed to 20 M cytochalasin B for 30 min, BHK-21 cell margins retracted, and the overall morphology became arborized (Fig. 10 D; see Goldman and Knipe, 1973). FRAP analyses showed complete but slower recovery with a t 1/2 of 11 5 min (n 12). Some bleach zones moved at m/min (n 7), a 60% decrease compared with controls, and squiggles continued to translocate (92/105 88%) at an average rate of m/min (n 17; range m/min; Fig. 10, D F), again similar to controls. Shape changes of fibrils and squiggles were seen at both concentrations of cytochalasin B (data not shown). The Motile Properties of IF Require Metabolic Energy To determine whether ATP is required for the motile properties of IF, we made time-lapse observations of GFPvimentin IF networks after treating BHK-21 cells with 50 mm 2-deoxy-d-glucose and 0.05% sodium azide for 30 min (Donaldson et al., 1990). None of the movements described above were detected over time periods of up to 40 min after 30 min of pretreatment with these inhibitors (Fig. 10, G H). These results demonstrate that the motile properties of vimentin IF require metabolic energy. Discussion The overall organization of IF networks containing GFPvimentin in several types of live cells during interphase, mitosis, and exposure to nocodazole were indistinguishable from those seen by immunofluorescence in nontransfected cells. Immunoblot and immunoprecipitation analyses of transfected cells indicated that a portion of endogenous vimentin formed stable complexes with the 82-kD GFP-vimentin. The nature of these complexes is unknown, but the solubilization conditions used for our immunoprecipitation assays suggest that these complexes represent dimers or tetramers containing endogenous and GFP-vimentin. These results, in addition to the FRAP observations, reassure us that the previously described properties of vimentin IF are retained after transfection, and that GFP-vimentin is a faithful reporter of the dynamic properties of IF in living cells. The Motile Properties of Vimentin IF Time-lapse observations of interphase cells expressing GFP-vimentin showed that fibrils exhibit numerous types of motility. For example, fibrils frequently appeared and disappeared in the same focal plane in the thin sheets of cytoplasm at the cell periphery. It is possible that these IF fibrils were moving in or out of the plane of focus. However, it is also possible that these observations represent local assembly or disassembly of IF (also see Prahlad et al., 1998). We also observed apparent fibril extension in the peripheral regions of cells. However, at the present time we cannot determine whether this extension of fibrils is due to active growth and elongation of fibrils or to the movement of preexisting fibrils due to sliding with respect to other cytoskeletal components such as MT and/or MF. Vimentin fibrils also appeared to be capable of lengthening and shortening as evidenced by the movement of interconnected foci either towards or away from each other. Movements of parallel arrays (not interconnected by foci) of vimentin fibrils were also observed due to the movements of bleach zones. Interestingly, both of these types of IF motility are sensitive to nocodazole and cytochalasin B, indicating a dependence on both MT and MF network integrity. It should be noted that there is evidence that the movement of IF towards the nucleus is MF dependent (Hollenbeck et al., 1989), and that their movement towards the cell surface is MT-dependent. The latter dependency appears to be related to a kinesin motor activity (Gyoeva and Gelfand, 1991; Prahlad et al., 1998). Short filamentous structures with two free ends, vimentin squiggles (also see Osborn, 1980; Prahlad et al., 1998), exhibited rapid movements, primarily towards the edge of cells at speeds up to 11 m/min. Squiggles continued to translocate at the same rates as controls in the presence of cytochalasin B. However, only 5% of the squiggles moved at the same rates as controls in the absence of MT. These observations suggest that translocation of the vast majority of squiggles is dependent on MT, most likely through MT-associated motor molecules such as kinesin. The observation that a small number of squiggles continue to translocate in the absence of MT is intriguing, and suggests that the motility of a subpopulation may be dependent on either MF and their associated proteins or unknown factors. In support of the former possibility, IF have been shown to interact with MF, and proteins have been identified that can form cross-bridges between these two cytoskeletal systems (Brown et al., 1995; McLean et al., 1996; Yang et al., 1996). It is also interesting to compare the rates of movement of bleach zones and photoactivated fluorescent marks along MT and MF with the rates obtained for IF in this study. For MT, analyses of mitotic and interphase MT bleach zones have revealed rates of m/min (Gorbsky and Borisy, 1989; Mitchison, 1989; Waterman- Storer and Salmon, 1997). Bleach zones made across microspikes containing fluorescent MF moved at m/min (Wang, 1985), and in stress fibers the rates were m/min (McKenna and Wang, 1986). Therefore, the rate of translocation of bleach zones on vimentin fibrils is similar to the rates recorded for MT and MF, irrespective of the differences in the mechanisms responsible for these movements (i.e., treadmilling for MT and MF). Intriguingly, adjacent vimentin fibrils move either towards or away from the cell center at different speeds while other fibrils in neighboring cytoplasmic regions remain stationary. These observations are different from the unidirectional movements of bleached zones toward the nucleus thus far reported for interphase MT and MF (Mc- Kenna and Wang, 1986; Waterman-Storer and Salmon, 1997). Vimentin fibrils frequently changed their shapes as evidenced by bending or straightening movements within short time intervals. These movements continued in the presence of nocodazole and cytochalasin B, suggesting that they represent intrinsic motile properties of IF independent of MT and MF. Interestingly, metabolic inhibitors completely arrested these shape changes, demonstrating that these movements require energy. Yoon et al. Motility of Intermediate Filaments 155
10 Use of GFP-vimentin To Monitor Subunit Exchange In Vivo Using GFP-vimentin in FRAP analyses has permitted us to calculate accurately a recovery half-time (t 1/2 ) of 5 3 min in BHK-21 cells. Similar analyses of interphase MT have indicated a t 1/2 of min in PtK1 cells, and min in BSC cells (Saxton et al., 1984). In the case of actin in stress fibers, a t 1/2 of 5 10 min has been reported (Kreis et al., 1982). Therefore, the recovery half-times for vimentin IF are very similar to those made for the other major cytoskeletal components. It is also interesting that the fluorescence recovery appeared to slow down relative to controls in the presence of MT and MF inhibitors. Therefore, the exchange mechanisms involved in the steady-state turnover of vimentin may depend to some unknown extent on either MT or MF. It is conceivable that in the case of MT, the movement of exchangeable vimentin subunits may be related to the kinesin-based motility of vimentin IF (Gyoeva and Gelfand, 1991) and the recently discovered protofilamentous aggregates of IF protein found in spreading fibroblasts (Prahlad et al., 1998). Type III IF networks containing vimentin exhibit a remarkable diversity of motile properties that require energy. The majority of these properties appear to reflect interactions with MT, MF, and their associated proteins (see Chou et al., 1997). Only the shape changes exhibited by IF appear to be independent of the other cytoskeletal systems. The mechanism underlying the changes in shape may be related to posttranslational modifications such as phosphorylation/dephosphorylation, which are thought to be involved in regulating the assembly and disassembly of IF (Eriksson et al., 1992; Inagaki et al., 1996). In this regard, it has been hypothesized that the hierarchical structure of IF produces a thermodynamically unstable seam that spirals along their length (Stewart, 1993). It is likely that this seam contains the most readily exchangeable subunits within IF. Perhaps the differential removal or addition of subunits in the region of this seam relative to more rigid regions of the IF wall could account for the changes in the shape of vimentin fibrils in vivo. In summary, GFP-vimentin fibrils can translocate, appear, or disappear within short time intervals, alter their shapes, and apparently alter their lengths. The significance of these motile properties of IF remains unknown. However, the surprisingly complex and continuous movements of intermediate filaments raise interesting new questions regarding their functions and their interactions with other cytoskeletal systems. We greatly appreciate the excellent technical assistance of Ms. Satya Khuon and Ms. Laura Davis for assistance in typing this manuscript. The work has been supported by the National Institute of General Medical Sciences and is in partial fulfillment of the Ph.D degree for M. Yoon. Received for publication 3 June 1998 and in revised form 26 August References Alber, K., and E. Fuchs Expression of mutant keratin cdnas in epithelial cells reveals possible mechanisms for initiation and assembly of intermediate filaments. J. Cell Biol. 108: Aubin, J.E., M. Osborn, W.W. Franke, and K. Weber Intermediate filaments of the vimentin-type and the cytokeratin-type are distributed differently during mitosis. Exp. Cell Res. 129: Brown, A., G. Bernier, M. Mathieu, J. Rossant, and R. Kothary The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat. Genet. 10: Chou, Y.H., J.R. Bischoff, D. Beach, and R.D. Goldman Intermediate filament reorganization during mitosis is mediated by p34 cdc2 phosphorylation of vimentin. Cell. 62: Chou, Y.H., P. Opal, R.A. Quinlan, and R.D. Goldman The relative roles of specific N- and C-terminal phosphorylation sites in the disassembly of intermediate filament in mitotic BHK-21 cells. J. Cell Sci. 109: Chou, Y.H., O. Skalli, and R.D. Goldman Intermediate filaments and cytoplasmic networking: new connections and more functions. Curr. Opin. Cell Biol. 9: Collier, N.C., M.P. Sheetz, and M.J. Schlesinger Concomitant changes in mitochondria and intermediate filaments during heat shock and recovery of chicken embryo fibroblasts. J. Cell Biochem. 52: Cubitt, A.B., R. Heim, S.R. Adams, A.E. Boyd, L.A. Gross, and R.Y. Tsien Understanding, improving and using green fluorescent proteins. Trends Cell Biol. 20: Donaldson, J.G., J. Lippincott-Schwartz, G.S. Bloom, T.E. Kreis, and R.D. Klausner Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J. Cell Biol. 111: Eriksson, J.E., D.L. Brautigan, R. Vallee, J. Olmsted, H. Fujiki, and R.D. Goldman Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc. Natl. Acad. Sci. USA. 89: Evan, G.I., G.K. Lewis, G. Ramsay, and J.M. Bishop Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5: Fischer, M., S. Kaech, D. Knutti, and A. Matus Rapid actin-based plasticity in dendritic spines. Neuron. 20: Franke, W.W., E. Schmid, C. Grund, and B. Geiger Intermediate filament proteins in nonfilamentous structures: transient disintegration and inclusion of subunit proteins in granular aggregates. Cell. 30: Goldman, R.D., and D.M. Knipe Functions of cytoplasmic fibers in nonmuscle cell motility. Cold Spring Harbor Symp. Quant. Biol. 37: Gorbsky, G.J., and G.G. Borisy Microtubules of the kinetochore fiber turn over in metaphase but not in anaphase. J. Cell Biol. 109: Gyoeva, F.K., and V.I. Gelfand Co-alignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature. 353: Hirokawa, N., S. Hisanaga, and Y. Shiomura MAP2 is a component of crossbridges between microtubules and neurofilaments in the neuronal cytoskeleton: quick-freeze, deep-etch immunoelectron microscopy and reconstitution studies. J. Neurosci. 8: Hollenbeck, P.J., A.D. Bershadsky, O.Y. Pletjushkina, I.S. Tint, and J.M. Vasiliev Intermediate filament collapse is an ATP-dependent and actindependent process. J. Cell Sci. 92: Ho, C.-L., J.L. Martys, A. Mikhailov, G.G. Gundersen, and R.K.H. Liem Novel features of intermediate filament dynamics revealed by green flourescent protein chimeras. J. Cell Sci. 111: Inagaki, M., Y. Matsuoka, K. Tsujimura, S. Ando, T. Tokui, T. Takahashi, and N. Inagaki Dynamic properties of intermediate filaments: regulation by phosphorylation. Bioessay. 18: Jones, S.M., J.C.R. Jones, and R.D. Goldman, Fractionation of desmosomes and comparison of the polypeptide composition of desmosomes prepared from two bovine epithelial tissues. J. Cell. Biochem. 36: Klymkowsky, M.W Intermediate filaments in 3T3 cells collapse after intracellular injection of a monoclonal anti-intermediate filament antibody. Nature. 291: Kreis, T.E., B. Geiger, and J. Schlessinger Mobility of microinjected rhodamine actin within living chicken gizzard cells determined by fluorescence photobleaching recovery. Cell. 29: Laemmli, U.K Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227: Liao, G., T. Nagasaki, and G.G. Gundersen Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level: implications for the role of dynamic microtubules in cell locomotion. J. Cell Sci. 108: Ludin, B., and A. Mauts GFP illuminates the cytoskeleton. Trends Cell Biol. 8: McKenna, N.M., and Y.L. Wang Possible translocation of actin and alpha-actinin along stress fibers. Exp. Cell Res. 167: McLean, W.H., L. Pulkkinen, F.J. Smith, E.L. Rugg, E.B. Lane, F. Bullrich, R.E. Burgeson, S. Amano, D.L. Hudson, K. Owaribe, J.A. McGrath, J.R. McMillan, R.A. Eady, I.M. Leigh, A.M. Christiano, and J. Uitto Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cdna cloning and genomic organization. Gene Dev. 10: Miller, R.K., S. Khuon, and R.D. Goldman Dynamics of keratin assembly: exogenous Type I keratin rapidly associates with Type II keratin in vivo. J. Cell Biol. 122: Mitchison, T.J Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J. Cell Biol. 109: Ngai, J., T.R. Coleman, and E. Lazarides Localization of newly synthesized vimentin subunits reveals a novel mechanism of intermediate filament assembly. Cell. 60: Okabe, S., H. Miyasaka, and N. Hirokawa Dynamics of the neuronal intermediate filaments. J. Cell Biol. 121: The Journal of Cell Biology, Volume 143,
11 Olson, K.R., J.R. McIntosh, and J.B. Olmsted Analysis of MAP 4 function in living cells using green fluorescent protein (GFP) chimeras. J. Cell Biol. 130: Osborn, M., W. Franke, and K. Weber Direct demonstration of the presence of two immunologically distinct intermediate-sized filament systems in the same cell by double immunofluorescence microscopy. Vimentin and cytokeratin fibers in cultured epithelial cells. Exp. Cell Res. 125: Prahlad, V., M. Yoon, R.D. Moir, R.D. Vale, and R.D. Goldman Rapid movements of vimentin on microtubule tracks: kinesin dependent assembly of Intermediate Filament networks. J. Cell Biol. 143: Rosevear, E.R., M. McReynolds, and R.D. Goldman Dynamic properties of intermediate filaments: disassembly and reassembly during mitosis in baby hamster kidney cells. Cell Motil. Cytoskelet. 17: Saxton, W.M., D.L. Stemple, R.J. Leslie, E.D. Salmon, M. Zavortink, and J.R. McIntosh Tubulin dynamics in cultured mammalian cells. J. Cell Biol. 99: Schliwa, M., and J. van Blerkom Structural interaction of cytoskeletal components. J. Cell Biol. 90: Soellner, P., R.A. Quinlan, and W.W. Franke Identification of a distinct soluble subunit of an intermediate filament protein: tetrameric vimentin from living cells. Proc. Natl. Acad. Sci. USA. 82: Steinert, P.M., W.W. Idler, and S.B. Zimmerman Self-assembly of bovine epidermal keratin filaments in vitro. J. Mol. Biol. 108: Stewart, M Intermediate filament structure and assembly. Curr. Opin. Cell Biol. 5:3 11. Svitkina, T.M., A.B. Verkhovsky, and G.G. Borisy Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J. Cell Biol. 135: Towbin, H., T. Staehelin, and J. Gordon Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 76: Vasquez, R.J., B. Howell, A.M. Yvon, P. Wadsworth, and L. Cassimeris Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell. 8: Vikstrom, K.L., G.G. Borisy, and R.D. Goldman Dynamic aspects of intermediate filament networks in BHK-21 cells. Proc. Natl. Acad. Sci. USA. 86: Vikstrom, K.L., S.S. Lim, R.D. Goldman, and G.G. Borisy Steady state dynamics of intermediate filament networks. J. Cell Biol. 118: Wang, Y.L Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J. Cell Biol. 101: Waterman-Storer, C.M., and E.D. Salmon Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J. Cell Biol. 139: Yang, H.Y., N. Lieska, A.E. Goldman, and R.D. Goldman A 300,000- mol-wt intermediate filament-associated protein in baby hamster kidney (BHK-21) cells. J. Cell Biol. 100: Yang, H.Y., N. Lieska, A.E. Goldman, and R.D. Goldman Colchicinesensitive and colchicine-insensitive intermediate filament systems distinguished by a new intermediate filament-associated protein, IFAP-70/280 kd. Cell Motil. Cytoskelet. 22: Yang, Y., J. Dowling, Q.C. Yu, P. Kouklis, D.W. Cleveland, and E. Fuchs An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell. 86: Zackroff, R.V., and R.D. Goldman In vitro assembly of intermediate filaments from baby hamster kidney (BHK-21) cells. Proc. Natl. Acad. Sci. USA. 76: Yoon et al. Motility of Intermediate Filaments 157
Intermediate Filaments in Motion: Observations of Intermediate Filaments in Cells Using Green Fluorescent Protein-Vimentin V
 Molecular Biology of the Cell Vol. 10, 1289 1295, May 1999 Intermediate Filaments in Motion: Observations of Intermediate Filaments in Cells Using Green Fluorescent Protein-Vimentin V Jayme L. Martys,
Molecular Biology of the Cell Vol. 10, 1289 1295, May 1999 Intermediate Filaments in Motion: Observations of Intermediate Filaments in Cells Using Green Fluorescent Protein-Vimentin V Jayme L. Martys,
CHAPTER 16 THE CYTOSKELETON
 CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
(phosphatase tensin) domain is shown in dark gray, the FH1 domain in black, and the
 Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Phalloidin-induced actin polymerization in the cytoplasm of
 Proc. Nati. Acad. Sci. USA Vol. 74, No. 12, pp. 5613-5617, December 1977 Cell Biology Phalloidin-induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth
Proc. Nati. Acad. Sci. USA Vol. 74, No. 12, pp. 5613-5617, December 1977 Cell Biology Phalloidin-induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth
The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit
 Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Rapid Movements of Vimentin on Microtubule Tracks: Kinesin-dependent Assembly of Intermediate Filament Networks
 Rapid Movements of Vimentin on Microtubule Tracks: Kinesin-dependent Assembly of Intermediate Filament Networks Veena Prahlad,* Miri Yoon,* Robert D. Moir,* Ronald D. Vale, and Robert D. Goldman* *Department
Rapid Movements of Vimentin on Microtubule Tracks: Kinesin-dependent Assembly of Intermediate Filament Networks Veena Prahlad,* Miri Yoon,* Robert D. Moir,* Ronald D. Vale, and Robert D. Goldman* *Department
Chapter 15. Cytoskeletal Systems. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
Microtubules. Forms a sheet of protofilaments that folds to a tube Both tubulins bind GTP
 Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
The following antibodies were used in this study: NuMA - rabbit-anti NuMA (gift from
 Materials and Methods Antibodies: The following antibodies were used in this study: NuMA - rabbit-anti NuMA (gift from D. A. Compton); Dynein Intermediate Chain - 74.1 (Chemicon, Temecula, CA); Dynein
Materials and Methods Antibodies: The following antibodies were used in this study: NuMA - rabbit-anti NuMA (gift from D. A. Compton); Dynein Intermediate Chain - 74.1 (Chemicon, Temecula, CA); Dynein
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
SUPPLEMENTARY INFORMATION FIGURE 1 - 1
 SUPPLEMENTARY INFORMATION FIGURE 1-1 SUPPLEMENTARY INFORMATION FIGURE 2-2 SUPPLEMENTARY INFORMATION METHODS GST-Pull-Down. Cultures of E. Coli (BL21) were transformed with pgex (Clontech) and pgex recombinant
SUPPLEMENTARY INFORMATION FIGURE 1-1 SUPPLEMENTARY INFORMATION FIGURE 2-2 SUPPLEMENTARY INFORMATION METHODS GST-Pull-Down. Cultures of E. Coli (BL21) were transformed with pgex (Clontech) and pgex recombinant
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non
 Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non 2. Plating density: sparse vs. confluent 3.
Variables in standard immunostaining protocol 2-step or indirect immunofluorescence 1. Substrate on which cells are plated: plastic vs. glass; coating vs. non 2. Plating density: sparse vs. confluent 3.
Biophysics of Molecules
 Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
The Organizational Fate of Intermediate Filament Networks in Two Epithelial Cell Types during Mitosis
 The Organizational Fate of Intermediate Filament Networks in Two Epithelial Cell Types during Mitosis JONATHAN C. R. JONES, ANNE E. GOLDMAN, HSI-YUAN YANG, and ROBERT D. GOLDMAN Department of Cell Biology
The Organizational Fate of Intermediate Filament Networks in Two Epithelial Cell Types during Mitosis JONATHAN C. R. JONES, ANNE E. GOLDMAN, HSI-YUAN YANG, and ROBERT D. GOLDMAN Department of Cell Biology
In vitro EGFP-CALI comprehensive analysis. Liang CAI. David Humphrey. Jessica Wilson. Ken Jacobson. Dept. of Cell and Developmental Biology
 In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
THE BASICS OF IMMUNOHISTOCHEMISTRY
 THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
Methanol fixation allows better visualization of Kal7. To compare methods for
 Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Supplementary Material
 Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Figure Legends
 Supplementary Figure Legends Figure S1 gene targeting strategy for disruption of chicken gene, related to Figure 1 (f)-(i). (a) The locus and the targeting constructs showing HpaI restriction sites. The
Supplementary Figure Legends Figure S1 gene targeting strategy for disruption of chicken gene, related to Figure 1 (f)-(i). (a) The locus and the targeting constructs showing HpaI restriction sites. The
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
SUPPLEMENTARY NOTE 3. Supplememtary Note 3, Wehr et al., Monitoring Regulated Protein-Protein Interactions Using Split-TEV 1
 SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or
 Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Organelle Atlas APPENDIX TO CHAPTER 4 4A.1
 APPENDIX TO CHAPTER 4 This atlas contains images of cellular organelles visualized using a wide variety of reagents and techniques and demonstrates the diversity of methods for exploring the cell. The
APPENDIX TO CHAPTER 4 This atlas contains images of cellular organelles visualized using a wide variety of reagents and techniques and demonstrates the diversity of methods for exploring the cell. The
Actin cytoskeleton of spread fibroblasts appears to assemble at the cell edges.j. Cell Sci. 82:
 See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/19367718 Actin cytoskeleton of spread fibroblasts appears to assemble at the cell edges.j.
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/19367718 Actin cytoskeleton of spread fibroblasts appears to assemble at the cell edges.j.
Cytoskeleton. B. Balen
 Cytoskeleton B. Balen structural backbone of the cell supports the fragile plasma membrane provides mechanical linkages that let the cell bear stresses without being ripped apart Importance and function
Cytoskeleton B. Balen structural backbone of the cell supports the fragile plasma membrane provides mechanical linkages that let the cell bear stresses without being ripped apart Importance and function
CELL BIOLOGY - CLUTCH CH CYTOSKELETON AND CELL MOVEMENT.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
!! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
October 24, BIOS 95: Cell Division and Chromosome Dynamics
 October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb
 Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
SUPPLEMENTAL MATERIAL. Supplemental Methods:
 SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
Supplementary Protocol. sirna transfection methodology and performance
 Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
SUPPLEMENTARY INFORMATION
 a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
CYTOSKELETON, MOTORPROTEINS.
 CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
I NTERMEDIAT~ filaments (IF) l are generally considered
 Steady State Dynamics of Intermediate Filament Networks Karen L. Vikstrom,* Soo-Siang Lira,* Robert D. Goldman,* and Gary G. Borisy~ * Department of Cell, Molecular, and Structural Biology, Northwestern
Steady State Dynamics of Intermediate Filament Networks Karen L. Vikstrom,* Soo-Siang Lira,* Robert D. Goldman,* and Gary G. Borisy~ * Department of Cell, Molecular, and Structural Biology, Northwestern
1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called.
 Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
Supplementary Figure 1. α-synuclein is truncated in PD and LBD brains. Nature Structural & Molecular Biology: doi: /nsmb.
 Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Section 10. Junaid Malek, M.D.
 Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
Fig. S1. Endocytosis of extracellular cargo does not depend on dia. (A) To visualize endocytosis, fluorescently labelled wheat germ agglutinin
 Fig. S1. Endocytosis of extracellular cargo does not depend on dia. (A) To visualize endocytosis, fluorescently labelled wheat germ agglutinin (WGA-Alexa555) was injected into the extracellular perivitteline
Fig. S1. Endocytosis of extracellular cargo does not depend on dia. (A) To visualize endocytosis, fluorescently labelled wheat germ agglutinin (WGA-Alexa555) was injected into the extracellular perivitteline
Supplemental Material
 Supplemental Material 1 Figure S1. Phylogenetic analysis of Cep72 and Lrrc36, comparative localization of Cep72 and Lrrc36 and Cep72 antibody characterization (A) Phylogenetic alignment of Cep72 and Lrrc36
Supplemental Material 1 Figure S1. Phylogenetic analysis of Cep72 and Lrrc36, comparative localization of Cep72 and Lrrc36 and Cep72 antibody characterization (A) Phylogenetic alignment of Cep72 and Lrrc36
T H E J O U R N A L O F C E L L B I O L O G Y
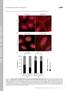 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
The cytoskeleton. The cytoskeleton, the motor proteins, the muscle and its regulation. The cytoskeleton. The cytoskeleton.
 , the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
, the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
Three major types of cytoskeleton
 The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
SUPPLEMENTARY INFORMATION FILE
 SUPPLEMENTARY INFORMATION FILE Existence of a microrna pathway in anucleate platelets Patricia Landry, Isabelle Plante, Dominique L. Ouellet, Marjorie P. Perron, Guy Rousseau & Patrick Provost 1. SUPPLEMENTARY
SUPPLEMENTARY INFORMATION FILE Existence of a microrna pathway in anucleate platelets Patricia Landry, Isabelle Plante, Dominique L. Ouellet, Marjorie P. Perron, Guy Rousseau & Patrick Provost 1. SUPPLEMENTARY
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Phosphorylation and disassembly of intermediate filaments
 Proc. Nati. Acad. Sci. USA Vol. 86, pp. 1885-1889, March 1989 Cell Biology Phosphorylation and disassembly of intermediate filaments in mitotic cells (vimentin/desmin/kinase) YNG-HAO CHOU, ELLEN ROSEVEAR,
Proc. Nati. Acad. Sci. USA Vol. 86, pp. 1885-1889, March 1989 Cell Biology Phosphorylation and disassembly of intermediate filaments in mitotic cells (vimentin/desmin/kinase) YNG-HAO CHOU, ELLEN ROSEVEAR,
Supplementary Figure S1. Np95 interacts with de novo methyltransferases Dnmt3a and 3b. (A) Co-immunoprecipitation of endogenous Dnmt3a2 (left and
 Supplementary Figure S1. Np95 interacts with de novo methyltransferases Dnmt3a and 3b. (A) Co-immunoprecipitation of endogenous Dnmt3a2 (left and right), Dnmt3b isoforms (left) and Dnmt1 (right) with GFP-Np95
Supplementary Figure S1. Np95 interacts with de novo methyltransferases Dnmt3a and 3b. (A) Co-immunoprecipitation of endogenous Dnmt3a2 (left and right), Dnmt3b isoforms (left) and Dnmt1 (right) with GFP-Np95
Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced
 Biophysical Journal, Volume 113 Supplemental Information Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced Kinesin-1 Motility Linda Balabanian, Christopher L. Berger, and Adam G. Hendricks
Biophysical Journal, Volume 113 Supplemental Information Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced Kinesin-1 Motility Linda Balabanian, Christopher L. Berger, and Adam G. Hendricks
De Novo Formation of Cytokeratin Filament Networks Originates From the Cell Cortex in A-431 Cells
 Cell Motility and the Cytoskeleton 50:33 44 (2001) De Novo Formation of Cytokeratin Filament Networks Originates From the Cell Cortex in A-431 Cells Reinhard Windoffer and Rudolf E. Leube* Department of
Cell Motility and the Cytoskeleton 50:33 44 (2001) De Novo Formation of Cytokeratin Filament Networks Originates From the Cell Cortex in A-431 Cells Reinhard Windoffer and Rudolf E. Leube* Department of
Chapter One. Construction of a Fluorescent α5 Subunit. Elucidation of the unique contribution of the α5 subunit is complicated by several factors
 4 Chapter One Construction of a Fluorescent α5 Subunit The significance of the α5 containing nachr receptor (α5* receptor) has been a challenging question for researchers since its characterization by
4 Chapter One Construction of a Fluorescent α5 Subunit The significance of the α5 containing nachr receptor (α5* receptor) has been a challenging question for researchers since its characterization by
Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and
 SUPPLEMENTARY MATERIALS AND METHODS Chromatin Immunoprecipitation for qpcr analysis Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and IL24, all located on chromosome 1. Primer
SUPPLEMENTARY MATERIALS AND METHODS Chromatin Immunoprecipitation for qpcr analysis Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and IL24, all located on chromosome 1. Primer
Molecular Cell Biology
 Molecular Cell Biology Biophysics of Helical Polymers Intermediate Filaments Michael Greenberg Department of Biochemistry and Molecular Biophysics greenberg@biochem.wustl.edu Intermediate Filaments Diversity
Molecular Cell Biology Biophysics of Helical Polymers Intermediate Filaments Michael Greenberg Department of Biochemistry and Molecular Biophysics greenberg@biochem.wustl.edu Intermediate Filaments Diversity
cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular processes (transcription, replication, microtubules)
 DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
Ab-DeliverIN TM - Antibody Delivery Reagent Results
 Ab-DeliverIN TM - Antibody Delivery Reagent Results OZ Biosciences is delighted to announce the launching of the innovative Ab-DeliverIN TM - antibody delivery reagent. Ab-DeliverIN TM is a lipid based
Ab-DeliverIN TM - Antibody Delivery Reagent Results OZ Biosciences is delighted to announce the launching of the innovative Ab-DeliverIN TM - antibody delivery reagent. Ab-DeliverIN TM is a lipid based
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
Supplementary Fig.1 Luton
 Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
Description of supplementary material file
 Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
ab CytoPainter ER Staining Kit Red Fluorescence
 ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
Cell culture. HeLa cells were cultured as monolayers in Dulbecco s Minimal Essential
 Supporting Online Material Materials and methods Cell culture. HeLa cells were cultured as monolayers in Dulbecco s Minimal Essential Medium (Gibco BRL, Invitrogen Corporation, Carlsbad, CA, USA), supplemented
Supporting Online Material Materials and methods Cell culture. HeLa cells were cultured as monolayers in Dulbecco s Minimal Essential Medium (Gibco BRL, Invitrogen Corporation, Carlsbad, CA, USA), supplemented
MCBII. Points this page
 1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
JCB Online Supplemental Material
 JCB Online Supplemental Material Schinzel et al. Results At least one positive charge at the very COOH terminus is needed for mitochondrial sorting It has been reported that two consecutive positive charges
JCB Online Supplemental Material Schinzel et al. Results At least one positive charge at the very COOH terminus is needed for mitochondrial sorting It has been reported that two consecutive positive charges
Cell lines and cell culture. GM08505 is an SV40-transformed skin fibroblast cell line
 Materials and Methods Cell lines and cell culture. GM08505 is an SV40-transformed skin fibroblast cell line derived from a BS patient and contains the BLM Ash founder mutation identified in patients of
Materials and Methods Cell lines and cell culture. GM08505 is an SV40-transformed skin fibroblast cell line derived from a BS patient and contains the BLM Ash founder mutation identified in patients of
Supplemental Information
 Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplementary Methods
 Supplementary Methods Antibodies For immunocytochemistry, the following antibodies were used: mouse anti-γ-h2ax (Upstate), rabbit anti-γ-h2ax (Abcam), rabbit anti-53bp1 (Novus), mouse anti-atm-phosphoserine1981
Supplementary Methods Antibodies For immunocytochemistry, the following antibodies were used: mouse anti-γ-h2ax (Upstate), rabbit anti-γ-h2ax (Abcam), rabbit anti-53bp1 (Novus), mouse anti-atm-phosphoserine1981
DCLK-immunopositive. Bars, 100 µm for B, 50 µm for C.
 Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Ono et al., http://www.jcb.org/cgi/content/full/jcb.201208008/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Chromatin-binding properties of MCM2 and condensin II during
Supplemental material Ono et al., http://www.jcb.org/cgi/content/full/jcb.201208008/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Chromatin-binding properties of MCM2 and condensin II during
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material THE JOURNAL OF CELL BIOLOGY Toso et al., http://www.jcb.org/cgi/content/full/jcb.200809055/dc1 Figure S1. Control experiments for sirna depletions used in Figs. 1 3. The phenotype
Supplemental Material THE JOURNAL OF CELL BIOLOGY Toso et al., http://www.jcb.org/cgi/content/full/jcb.200809055/dc1 Figure S1. Control experiments for sirna depletions used in Figs. 1 3. The phenotype
PROTOCOL. Live Cell Imaging of 3D Cultures Grown on Alvetex Scaffold Using Confocal Microscopy. Introduction
 Page 1 Introduction Live cell imaging allows real time monitoring of cell shape and form during cell differentiation, proliferation and migration. Alvetex Scaffold offers a unique ability to study cell
Page 1 Introduction Live cell imaging allows real time monitoring of cell shape and form during cell differentiation, proliferation and migration. Alvetex Scaffold offers a unique ability to study cell
Supporting Online Material Material and Methods
 Supporting Online Material Material and Methods Live-cell DIC recordings PtK 1 cells (ATCC, Manassas, VA) were filmed on a Nikon (Nikon Instruments, Melville, NY) inverted microscope equipped with 60x
Supporting Online Material Material and Methods Live-cell DIC recordings PtK 1 cells (ATCC, Manassas, VA) were filmed on a Nikon (Nikon Instruments, Melville, NY) inverted microscope equipped with 60x
Supplementary Information for. Regulation of Rev1 by the Fanconi Anemia Core Complex
 Supplementary Information for Regulation of Rev1 by the Fanconi Anemia Core Complex Hyungjin Kim, Kailin Yang, Donniphat Dejsuphong, Alan D. D Andrea* *Corresponding Author: Alan D. D Andrea, M.D. Alan_dandrea@dfci.harvard.edu
Supplementary Information for Regulation of Rev1 by the Fanconi Anemia Core Complex Hyungjin Kim, Kailin Yang, Donniphat Dejsuphong, Alan D. D Andrea* *Corresponding Author: Alan D. D Andrea, M.D. Alan_dandrea@dfci.harvard.edu
Supplemental Online Material. The mouse embryonic fibroblast cell line #10 derived from β-arrestin1 -/- -β-arrestin2 -/-
 #1074683s 1 Supplemental Online Material Materials and Methods Cell lines and tissue culture The mouse embryonic fibroblast cell line #10 derived from β-arrestin1 -/- -β-arrestin2 -/- knock-out animals
#1074683s 1 Supplemental Online Material Materials and Methods Cell lines and tissue culture The mouse embryonic fibroblast cell line #10 derived from β-arrestin1 -/- -β-arrestin2 -/- knock-out animals
ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry of cytochrome c and mitochondria.
 PROTOCOL ApoTrack Cytochrome c Apoptosis ICC Antibody Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSA07 Rev.1 DESCRIPTION ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry
PROTOCOL ApoTrack Cytochrome c Apoptosis ICC Antibody Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSA07 Rev.1 DESCRIPTION ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry
Cdc42 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
Supplemental Information: Phosphorylation of CLIP-170 by Both Plk1 and CK2 Is Involved in the Timely Formation of Kinetochore-microtubule Attachments
 Supplemental Information: Phosphorylation of CLIP-170 by Both Plk1 and CK2 Is Involved in the Timely Formation of Kinetochore-microtubule Attachments Hongchang Li, X. Shawn Liu, Xiaoming Yang, Yingmin
Supplemental Information: Phosphorylation of CLIP-170 by Both Plk1 and CK2 Is Involved in the Timely Formation of Kinetochore-microtubule Attachments Hongchang Li, X. Shawn Liu, Xiaoming Yang, Yingmin
Chromosome Structural Analysis
 Chromosome Structural Analysis A Practical Approach Edited by WENDY A. BICKMORE Cell Genetics Section, MRC Human Genetics Unit, Western General Hospital, Edinburgh OXJORD UNIVERSITY PRESS 1999 List of
Chromosome Structural Analysis A Practical Approach Edited by WENDY A. BICKMORE Cell Genetics Section, MRC Human Genetics Unit, Western General Hospital, Edinburgh OXJORD UNIVERSITY PRESS 1999 List of
Histone H3 Methylation Antibody Panel Pack I - Active Genes Base Catalog # C Component Size Shipping Temperature
 Histone H3 Methylation Antibody Panel Pack I - Active Genes Base Catalog # PACK CONTENTS Component Size Shipping Temperature Upon Receipt Checklist 3K4D Histone H3K4me2 (H3K4 Dimethyl) Polyclonal Antibody
Histone H3 Methylation Antibody Panel Pack I - Active Genes Base Catalog # PACK CONTENTS Component Size Shipping Temperature Upon Receipt Checklist 3K4D Histone H3K4me2 (H3K4 Dimethyl) Polyclonal Antibody
Immunofluorescence images of different core histones and different histone exchange assay.
 Molecular Cell, Volume 51 Supplemental Information Enhanced Chromatin Dynamics by FACT Promotes Transcriptional Restart after UV-Induced DNA Damage Christoffel Dinant, Giannis Ampatziadis-Michailidis,
Molecular Cell, Volume 51 Supplemental Information Enhanced Chromatin Dynamics by FACT Promotes Transcriptional Restart after UV-Induced DNA Damage Christoffel Dinant, Giannis Ampatziadis-Michailidis,
Gα i Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
CytoPainter Golgi Staining Kit Green Fluorescence
 ab139483 CytoPainter Golgi Staining Kit Green Fluorescence Instructions for Use Designed for the detection of Golgi bodies by microscopy This product is for research use only and is not intended for diagnostic
ab139483 CytoPainter Golgi Staining Kit Green Fluorescence Instructions for Use Designed for the detection of Golgi bodies by microscopy This product is for research use only and is not intended for diagnostic
ApoTrack Cytochrome c Apoptosis ICC Antibody Kit
 ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Kit Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and non-apoptotic
ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Kit Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and non-apoptotic
A guide to selecting control, diluent and blocking reagents
 Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
A guide to selecting control, diluent and blocking reagents
 Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Figure S2. Verification of ihog and boi
 Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
Supplementary methods
 Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
Chapter 3. DNA Replication & The Cell Cycle
 Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
RheB Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based RheB Activation Assay Kit Catalog Number: 81201 20 assays NewEast Biosciences 1 FAX: 610-945-2008 Table
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based RheB Activation Assay Kit Catalog Number: 81201 20 assays NewEast Biosciences 1 FAX: 610-945-2008 Table
Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by
 Supplementary Methods and Figures Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by methylene blue for Alzheimer s disease treatment Wenchao Sun 1, Seongsoo Lee 1,2, Xiaoran
Supplementary Methods and Figures Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by methylene blue for Alzheimer s disease treatment Wenchao Sun 1, Seongsoo Lee 1,2, Xiaoran
Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in
 Supplementary information Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer s disease Zhentao Zhang, Mingke Song, Xia Liu, Seong Su Kang, Il-Sun Kwon, Duc
Supplementary information Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer s disease Zhentao Zhang, Mingke Song, Xia Liu, Seong Su Kang, Il-Sun Kwon, Duc
42 fl organelles = 34.5 fl (1) 3.5X X 0.93 = 78,000 (2)
 SUPPLEMENTAL DATA Supplementary Experimental Procedures Fluorescence Microscopy - A Zeiss Axiovert 200M microscope equipped with a Zeiss 100x Plan- Apochromat (1.40 NA) DIC objective and Hamamatsu Orca
SUPPLEMENTAL DATA Supplementary Experimental Procedures Fluorescence Microscopy - A Zeiss Axiovert 200M microscope equipped with a Zeiss 100x Plan- Apochromat (1.40 NA) DIC objective and Hamamatsu Orca
Gα 13 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
Supplementary Figure 1 Collision-induced dissociation (CID) mass spectra of peptides from PPK1, PPK2, PPK3 and PPK4 respectively.
 Supplementary Figure 1 lision-induced dissociation (CID) mass spectra of peptides from PPK1, PPK, PPK3 and PPK respectively. % of nuclei with signal / field a 5 c ppif3:gus pppk1:gus 0 35 30 5 0 15 10
Supplementary Figure 1 lision-induced dissociation (CID) mass spectra of peptides from PPK1, PPK, PPK3 and PPK respectively. % of nuclei with signal / field a 5 c ppif3:gus pppk1:gus 0 35 30 5 0 15 10
Supplementary data. sienigma. F-Enigma F-EnigmaSM. a-p53
 Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
However, only a fraction of these genes are transcribed in an individual cell at any given time.
 All cells in an organism contain the same set of genes. However, only a fraction of these genes are transcribed in an individual cell at any given time. It is the pattern of gene expression that determines
All cells in an organism contain the same set of genes. However, only a fraction of these genes are transcribed in an individual cell at any given time. It is the pattern of gene expression that determines
