p27 kip1 independently promotes neuronal differentiation and migration in the cerebral cortex
|
|
|
- Gertrude Lauren Fox
- 6 years ago
- Views:
Transcription
1 p27 kip1 independently promotes neuronal differentiation and migration in the cerebral cortex Laurent Nguyen, 1 Arnaud Besson, 2 Julian Ik-Tsen Heng, 1 Carol Schuurmans, 3 Lydia Teboul, 1,5 Carlos Parras, 1 Anna Philpott, 4 James M. Roberts, 2 and François Guillemot 1,6 1 Division of Molecular Neurobiology, National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, United Kingdom; 2 Howard Hughes Medical Institute, Fred Hutchinson Cancer Research Center, Division of Basic Sciences, Seattle, Washington 98109, USA; 3 Department of Biochemistry and Molecular Biology, Institute of Maternal and Child Health, University of Calgary, Calgary, Alberta T2N 4N1, Canada; 4 Department of Oncology, Cambridge University, Hutchison/MRC Research Centre, Addenbrookes Hospital, Cambridge CB22XZ, United Kingdom The generation of neurons by progenitor cells involves the tight coordination of multiple cellular activities, including cell cycle exit, initiation of neuronal differentiation, and cell migration. The mechanisms that integrate these different events into a coherent developmental program are not well understood. Here we show that the cyclin-dependent kinase inhibitor p27 Kip1 plays an important role in neurogenesis in the mouse cerebral cortex by promoting the differentiation and radial migration of cortical projection neurons. Importantly, these two functions of p27 Kip1 involve distinct activities, which are independent of its role in cell cycle regulation. p27 Kip1 promotes neuronal differentiation by stabilizing Neurogenin2 protein, an activity carried by the N-terminal half of the protein. p27 Kip1 promotes neuronal migration by blocking RhoA signaling, an activity that resides in its C-terminal half. Thus, p27 Kip1 plays a key role in cortical development, acting as a modular protein that independently regulates and couples multiple cellular pathways contributing to neurogenesis. [Keywords: Neurogenesis; neurogenin; radial migration; RhoA; electroporation; RNA interference] Supplemental material is available at Received December 21, 2005; revised version accepted April 6, During development of the vertebrate CNS, cycling progenitors generate post-mitotic precursors that rapidly migrate out of germinal zones and initiate neuronal differentiation. The key steps of cell cycle exit, cell migration, and neuronal differentiation are tightly linked during neurogenesis. Proliferation and differentiation are largely incompatible cellular states in the CNS, and cells that initiate neuronal differentiation while remaining mitotic often die (Lee et al. 1992). Conversely, neuronal differentiation and neuronal migration involve a common process of cytoskeleton remodeling and are usually coupled (Luo 2000). A number of molecules that play important roles in regulating individual steps of neurogenesis, including cell cycle exit (Ohnuma and Harris 2003), cell migration (Bielas et al. 2004), and neuronal differentiation (Bertrand et al. 2002) have been identified. Little is known, however, of how these different cellular steps are coordinated in a coherent program of neurogenesis. 5 Present address: Gene Targeting and Transgenic Unit, Mary Lyon Center, Medical Research Council, Harwell, Oxfordshire OX11 0RD, UK. 6 Corresponding author. Fguille@nimr.mrc.ac.uk; FAX Article published online ahead of print. Article and publication date are online at Cyclin-dependent kinase inhibitors (CKIs) are good candidates to regulate multiple aspects of neurogenesis. Two families of CKIs promote cell cycle withdrawal by blocking the activity of cyclin/cyclin-dependent kinase (CDK) complexes: the Cip/Kip family, including p21 Cip1, p27 Kip1, and p57 Kip2, and the INK4 family, including p15 Ink4b, p16 Ink4a, p18 Ink4c, and p19 Ink4d (Elledge and Harper 1994). INK4 proteins act by inhibiting the activity of CDK4 and CDK6. Cip/Kip proteins have broader activities, as they interact with all cyclin/cdk complexes (Sherr and Roberts 1999). CKIs play an essential role in regulating cell cycle in neural tissues. In particular, p27 Kip1 has been implicated in promoting cell cycle arrest of neural progenitors during embryogenesis (Fero et al. 1996; Kiyokawa et al. 1996; Nakayama et al. 1996; Carruthers et al. 2003), in regulating the division of transit amplifying progenitors in the adult subventricular zone (Doetsch et al. 2002), and, together with p19 Ink4d, in maintaining differentiated neurons in a nonmitotic state (Zindy et al. 1999). Interestingly, there is accumulating evidence that Cip/ Kip proteins have activities that go beyond their wellcharacterized control of cell division. The three Cip/Kip proteins have been shown to regulate differentiation of GENES & DEVELOPMENT 20: by Cold Spring Harbor Laboratory Press ISSN /06;
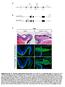
2 Nguyen et al. muscle cells (Zhang et al. 1999; Vernon and Philpott 2003) and white blood cells (Casini and Pelicci 1999; Steinman 2002). Cip/Kip proteins have also been implicated in fate specification and differentiation of glial cells, including oligodendrocytes (Durand et al. 1997; Zezula et al. 2001) and retinal Müller glia cells (Ohnuma et al. 1999). Less is known, however, of the role of these factors in neuronal differentiation, although p27 Kip1 has been implicated in primary neurogenesis in Xenopus embryos (Vernon et al. 2003), and p21 Cip1 has been shown to regulate neurite outgrowth in retinal cells (Tanaka et al. 2002). p27 Kip1 also appears to be an important regulator of cell migration in a variety of cell culture models, including fibroblasts, vascular smooth muscle cells, and endothelial cells (Sun et al. 2001; Diez-Juan and Andres 2003; McAllister et al. 2003). p27 Kip1 promotes migration of fibroblasts by blocking the activity of the small GTPase RhoA, and absence of p27 Kip1 results in increased number of stress fibers and focal adhesions, and reduced cell motility (Besson et al. 2004). Whether p27 Kip1 regulates cell migration in vivo, in particular in the nervous system, has not yet been addressed. The embryonic cortex is an excellent model to study how cell cycle exit, differentiation, and migration are coordinately regulated during neurogenesis. Cortical projection neurons are generated over a 7-d period in the mouse, from progenitor cells located in the germinal zone of the dorsal telencephalon. Newborn neurons migrate radially to reach the cortical plate, where they settle in distinct neuronal layers. Early-born neurons occupy deep cortical layers while later born neurons occupy progressively more superficial layers, resulting in an inside-out pattern of cortical histogenesis (Sidman and Rakic 1973). p27 Kip1 has been shown to play an important role in development of the cerebral cortex, by controlling the birth date of cortical neurons. In p27 Kip1 - null mutant mice, there is a decrease in neuronal production during mid-corticogenesis and an increase in production of late-born neurons, resulting in an enlargement of upper cortical layers (Goto et al. 2004). Conversely, overexpression of p27 Kip1 in cortical progenitors results in a reduction in number of upper layer neurons (Tarui et al. 2005). p27 Kip1 expression levels in cortical progenitors appear to determine both cell cycle length and the probability of cell cycle re-entry, and differences in p27 Kip1 expression levels between areas of the developing primate cortex have been implicated in area-specific levels of neuronal production (Lukaszewicz et al. 2005). Here, we have asked whether p27 Kip1 regulates aspects of cortical neurogenesis other than neuronal production. By analyzing p27 Kip1 -null mutant embryos and performing overexpression and knockdown experiments, we have shown that p27 Kip promotes both the radial migration and differentiation of newborn cortical neurons. These activities are cell cycle-independent and are independently regulated by distinct domains of the p27 Kip1 protein. Altogether, our results demonstrate that p27 Kip1 is a modular protein that regulates multiple pathways during neurogenesis and thereby plays a key role in coordinating cell cycle exit, differentiation, and radial migration during cortical development. Results p27 Kip is the predominant Cip/Kip protein in cortical progenitors and neurons To investigate the role of Cip/Kip proteins in cortical neurogenesis, we first examined the expression of p21 Cip1, p27 Kip1, and p57 Kip2 by RNA in situ hybridization and immunocytochemistry in embryonic day 14.5 (E14.5) mouse cortex. Only p27 Kip1 transcripts were detected at a significant level in the ventricular zone (VZ), subventricular zone (SVZ), and intermediate zone (IZ), while transcripts for all three genes were present in the cortical plate (CP) (Fig. 1A C). Similarly, p27 Kip1 is the only Cip/Kip protein expressed throughout the cerebral cortex, at a moderate level in a subset of VZ progenitors Figure 1. p27 Kip1 is expressed in cortical progenitors and neurons. (A C) In situ RNA hybridization with p21 Cip1, p27 Kip1, and p57 Kip2 probes on coronal sections through E14.5 dorso-lateral cortex. The three genes are expressed at low (p21 Cip1 ) or high levels (p27 Kip1, p57 Kip2 ) in the cortical plate (CP), while only p27 Kip1 transcripts are significantly present in the ventricular zone (VZ), subventricular zone (SVZ), and intermediate zone (IZ). (D F) Immunofluorescent staining at the same stage reveals a pattern of Cip/Kip protein expression comparable to that of their transcripts, with p27 Kip1 highly expressed from the SVZ to the CP and at lower level in the VZ, while p21 Cip1 and p57 Kip2 are only expressed at low levels in the CP. (G I) Note the predominantly cytoplasmic localization of p27 Kip1 in VZ and IZ cells and its nuclear localization in CP cells GENES & DEVELOPMENT
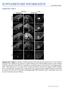
3 Multiple functions of p27 in neurogenesis and at elevated levels in neurons migrating through the IZ and into the CP (Fig. 1E). p27 Kip1 expression switches from predominantly cytoplasmic in VZ progenitors and IZ neurons to a predominantly nuclear localization in CP neurons (Fig. 1G I). p21 Cip1 and p57 Kip2 proteins were detected at low levels in a subset of CP cells (Fig. 1D,F). p27 Kip1 is therefore the predominant Cip/Kip protein in the E14.5 cerebral cortex. p27 Kip1 mutant mice exhibit defects in cortical neuron migration and differentiation that are unrelated to cell cycle exit deficits Given the broad cortical expression of p27 Kip1, including in post-mitotic neurons, we asked whether p27 Kip1 may have additional roles in cortical development beyond its well-established function in promoting cell cycle exit (Caviness et al. 2003). We first examined the radial migration of newly born neurons in p27 Kip1 -null mutant embryos (p27 / ) by conducting birth-dating experiments with bromodeoxyuridine (BrdU). Pregnant females were injected with a single dose of BrdU at E14.5, and embryos were harvested at E17.5. Brightly labeled BrdU-positive cells (neurons born at E14.5) were aberrantly distributed in the cortex of E17.5 p27 / embryos (Fig. 2A). A significantly greater number of BrdU-labeled cells accumulated in the VZ/SVZ and the IZ, and fewer cells reached the CP than in cortices from wild-type littermates (Fig. 2A). To determine if this defect in cortical neuron migration was related to the cell cycle regulation function of p27 Kip1, we examined cortical neuron migration in embryos homozygous for a mutant version of p27 Kip1 that no longer binds to cyclins and CDKs and does not promote cell cycle exit (p27 CK allele; Besson et al. 2006). Interestingly, no defect in the distribution of BrdU-labeled cells was observed in E17.5 p27 CK cortices injected with BrdU at E14.5 (Fig. 2A). Thus p27 Kip1 is required for the normal migration of cortical neurons, and this function is independent of its role in promoting cell cycle exit. Cortical neurons begin to express the neuronal differentiation marker HuC/D soon after exiting the cell cycle and before they migrate out of the VZ/SVZ (Fig. 2B). To determine whether p27 Kip1 also regulates the differentiation of cortical neurons, we examined the expression of HuC/D in the same embryos as above. The cortex of p27 / embryos showed a reduced number of E14.5-born neurons (BrdU-labeled) that expressed HuC/D at E17.5 compared with p27 CK embryos and wild-type littermates (Fig. 2B). Most of the reduction in neuronal differentiation was observed in the VZ/SVZ (44.8% ± 5.1% HuC/D+ cells among BrdU+ cells in the VZ/SVZ of p27 / embryos, compared with 81.3% ± 3.5% in wild- Figure 2. p27 Kip1 -null mutant cortices present defects in neuronal differentiation and radial migration. Pregnant females carrying p27 Kip1 mutant embryos were injected with a single dose of BrdU at E14.5 of gestation to mark neurons born at that date, and embryos were harvested at E17.5 and analyzed with antibodies to BrdU and HuC/D. (A) Loss of p27 Kip1 reduces radial migration of BrdU + cortical neurons, as shown by the accumulation of labeled cells in the VZ/SVZ and IZ and the loss of labeled cells in the CP of p27 / mutant embryos compared to wild-type littermates. Embryos homozygous for the cell cycle mutant allele p27 ck do not present defects in distribution of BrdU + neurons. The histogram shows the distribution of BrdU + cells in the different compartments of the cortex (VZ/SVZ, IZ, and CP) as a way to quantify radial migration in the different genotypes. Asterisks indicate significant differences in percentages of BrdU + cells in a given cortical zone in mutant and wild-type cortices (n =2 3, *p < 0.05, **p < 0.01). Panels on the right illustrate the distribution of BrdU + cortical cells in the different genotypes. (B) Loss of p27 Kip1 also reduces the differentiation of BrdU + cortical neurons, as marked by expression of HuC/D, while p27 CK embryos do not present this defect. The histogram shows the percentage of BrdU + cells expressing HuC/D in the different genotypes (n =2 3 brains; **p < 0.01). Panels illustrate double immunostaining for BrdU (green) and HuC/D (red). Arrows in insets indicate double-labeled cells. GENES & DEVELOPMENT 1513
4 Nguyen et al. type littermates and 82% ± 3.8% in p27 CK cortices; data not shown). Our results thus indicate that p27 Kip1 is required for the normal differentiation and migration of cortical cells and that these activities are cell cycle-independent. p27 Kip1 is the only Cip/Kip protein that promotes cortical neuron differentiation and migration We next asked whether all members of the Cip/Kip family shared the same cell cycle-independent activities in the cortex. For this, we performed overexpression experiments by in utero electroporation of cortices at E14.5 and analyzed electroporated cells and their progeny at E17.5 (Fig. 3A). To determine whether any activity of Cip/Kip proteins is dependent on their cell cycle regulatory function, we used both wild-type and cell cycle mutant versions of these proteins (Supplementary Fig. 1; Welcker et al. 1998; Ohnuma et al. 1999). Strikingly, overexpression of p27 Kip1 together with GFP enhanced the radial migration of electroporated cells away from the VZ/SVZ, resulting in an increased number of GFP + cells accumulating in the CP compared to control electroporated cells (Fig. 3B D). p27 Kip1 was the only CKI promoting cell migration in this assay (Fig. 3B). Conversely, radial migration was impaired when p27 Kip1 expression was acutely down-regulated by electroporation of two distinct sirnas, similar to the phenotype observed in the p27 / cortex, while a control sirna had no activity (Supplementary Fig. 2A,B). The radial glia scaffold was unaffected by p27 Kip1 knockdown, indicating a cell autonomous role for p27 Kip1 in radial migration (Supplementary Fig. 2C). Regulation of cell migration by p27 Kip1 was independent of its cell cycle activity since (1) the cell cycle mutant version of p27 Kip1 (p27 ck ) promoted radial migration as efficiently as wild-type p27 Kip1 (Fig. 3B,E) and (2) p21 Cip1 and p57 Kip2 had no activity on radial cell migration (Fig. 3B), although they promote cell cycle exit as efficiently as p27 Kip1 (Supplementary Fig. 1B). In addition to its effect on cell migration, overexpression of p27 Kip1 also produced an increased number of GFP + cells expressing HuC/D or III-tubulin compared to GFP control (Fig. 3F H). This effect of p27 Kip1 on differentiation was mostly observed in the VZ/SVZ, where undifferentiated progenitors cells reside (47.8% ± 1.3% HuC/D + cells and 34.9% ± 5.7% III-tubulin + cells among p27 Kip1 electroporated cells in the VZ/SVZ, compared with 27.3% ± 3.3% and 18.3% ± 1.6% in control experiments, respectively; data not shown). Conversely, knockdown of p27 Kip1 expression resulted in a reduction of HuC/D and III-tubulin expression, compared to GFP control (Supplementary Fig. 2D,E). Notably, only p27 Kip1 among the Cip/Kip genes promoted neuronal differentiation, and this activity was independent of its role in cell cycle regulation, since the cell cycle mutant form p27 ck was as efficient as wild-type p27 Kip1 at inducing expression of neuronal markers (Fig. 3F H). To demonstrate that the increased number of differentiated neurons observed upon p27 Kip1 overexpression resulted from premature differentiation of VZ/SVZ progenitors rather than a change in cell fate (e.g., from glial to neuronal), we measured both neuronal ( III-tubulin + ) and progenitor (nestin + ) populations among electroporated (GFP + ) cells by cultivating E14.5 electroporated cortices as slices for 2 d, followed by acute dissociation and analysis (see Materials and Methods). p27 wt overexpression resulted in an increase in III-tubulin + neurons and a parallel decrease in nestin + progenitors, demonstrating that it accelerates neuronal differentiation (Supplementary Fig. 3). A similar effect was obtained with the cell cycle mutant p27 ck. Conversely, p27 Kip1 knockdown led to a significant reduction of III-tubulin + neurons and to an increase in nestin + cells (Supplementary Fig. 3). To rule out that differences in radial migration and differentiation involved induction of cell death, we examined apoptotic cells with an antibody to activated caspase-3. The percentage of apoptotic cells among electroporated cells was very low (<1%) and similar with all constructs tested (data not shown). Taken together, these observations demonstrate that p27 Kip1 promotes both the differentiation of VZ/SVZ progenitors into neurons and the radial migration of neurons into the CP. These functions are unique to p27 Kip1 among the Cip1/Kip1 genes and are independent of its cell cycle regulatory function. p27 Kip1 is required at different times for radial migration and differentiation p27 Kip1 is expressed throughout the developing cerebral cortex, but its effects on neuronal differentiation and migration mostly take place in VZ/SVZ cells, as shown by changes in the fraction of cells migrating out of the VZ/ SVZ and in the fraction of VZ/SVZ cells expressing neuronal markers when p27 Kip1 is up- or down-regulated (Figs. 2, 3). To determine whether p27 Kip1 acts in VZ/ SVZ cells that are still cycling or have exited the cell cycle, we manipulated p27 Kip1 expression by ex vivo electroporation followed by organotypic slice culture (Hand et al. 2005) and monitored the proliferation status of electroporated cells by continuous BrdU exposure during the culture period (Supplementary Fig. 4A). Cell migration reached similar levels when E14.5 cortices were electroporated ex utero and cultivated in slices for 4 d, or electroporated in utero and harvested after 3 d, both in control conditions and following p27 wt or p27 ck overexpression and p27 Kip1 knockdown (cf. Supplementary Fig. 4B and Fig. 3B). Neuronal differentiation was slightly reduced in the slice culture assay compared with an in utero electroporation experiment in control conditions, but overexpression of p27 wt or p27 ck and p27 Kip1 knockdown affected neuronal differentiation to the same extent in the two assays (Fig. 3F,G; Supplementary Fig. 4C). The slice culture assay is therefore a valid approach to study p27 Kip1 function in neuronal differentiation and radial migration in the cortex. When electroporated cortices were cultivated for 4din the continuous presence of BrdU, a fraction of GFP + cells remained BrdU-negative, indicating that these cells had 1514 GENES & DEVELOPMENT
5 Multiple functions of p27 in neurogenesis Figure 3. p27 Kip1 overexpression promotes differentiation and radial migration of cortical neurons. (A) Coronal section through an E17.5 mouse brain electroporated in utero with a GFP construct at E14.5. The inset shows GFP fluorescence in the dorso-lateral cortex in the same brain. Three days after electroporation, the progeny of GFP + electroporated cells (green) were distributed in all zones of the developing cortex (marked by TOTO-3 in red) indicating that electroporated cells have undergone radial migration. (B) Distribution of GFP + cells in the different zones of the cortex at E17.5, following in utero electroporation at E14.5 of various bicistronic constructs expressing a Cip/ Kip protein and GFP. p27 Kip1 is the only Cip/Kip gene that promotes radial cell migration when overexpressed, and this effect is independent of cell cycle regulation as the cell cycle mutant form p27 ck is as efficient as the wild-type version p27 wt. Wild-type p21 Cip1 and p57 Kip2 (p21 wt and p57 wt ) and mutant versions that lack cell cycle regulation activity (p21 ck and p57 ck ) lack radial migration activity (n =3 5 brains; *p < 0.05; **p < 0.01). (C E) Immunostaining for GFP illustrating the position of cells electroporated with GFP, p27 wt,orp27 ck.(f H) Differentiation of cortical cells electroporated in utero at E14.5 and analyzed at E17.5 for expression of the neuronal differentiation markers HuC/D (F,H) and III-tubulin (G). Overexpression of p27 Kip1 promotes expression of neuronal markers independently of its role in cell cycle regulation, as its action is mimicked by p27 ck, while overexpression of wild-type or cell cycle mutant forms of p21 Cip1 or p57 Kip2 does not significantly promote neuronal differentiation. The histograms show the percentage of GFP + Hu + (F) and GFP + III-tubulin + (G) double-labeled cells over the total population of GFP + electroporated cells (n =3 5 brains; **p < 0.01). (H) Double immunostaining for GFP (green) and HuC/D (red) illustrating the induction of neuronal differentiation by p27 wt and p27 ck. Arrows in insets indicate double-labeled cells. Bars: C E, 50 µm. passed the last S phase but still had a ventricular process at the time of electroporation, while the remaining GFP + cells were BrdU + and had therefore passed through S phase at least once during the culture period (Supplementary Fig. 4A). When p27 wt or p27 ck -electroporated cortices were cultivated in the presence of BrdU, there was a highly significant increase of migration among the BrdU cell population. p27 Kip1 knockdown reciprocally induced a decrease in migration among BrdU cells, suggesting that p27 Kip1 exerts its migration activity on VZ cells that have become post-mitotic (Supplementary Fig. 4D,F). In contrast, p27 Kip1 knockdown significantly reduced HuC/D expression only in the cycling (BrdU + ) cohort of electroporated cells and not in the population of GENES & DEVELOPMENT 1515
6 Nguyen et al. electroporated cells that were exiting the cell cycle (BrdU ) (Supplementary Fig. 4E,F). This suggests that p27 Kip1 is required before or during the last S phase of VZ precursors for their differentiation while it is required after the last S phase for their migration. Thus, p27 Kip1 may regulate cell differentiation and cell migration by different mechanisms, operating at different times in the cell cycle. p27 Kip1 regulates Ngn2 expression by stabilizing Ngn2 protein To further address the possibility that p27 Kip1 independently regulates cell migration and neuronal differentiation in the cortex, we examined the molecular mechanisms underlying these cell cycle-independent functions. The proneural basic helix loop helix (bhlh) gene Ngn2 plays a central role in cortical neurogenesis, specifying cortical progenitors to the neuronal fate, inducing a glutamatergic pyramidal neuron phenotype and promoting the radial migration of cortical neurons (Nieto et al. 2001; Schuurmans et al. 2004; Hand et al. 2005). We thus asked whether p27 Kip1 might exert its cell cycle-independent activities in the cortex by regulating Ngn2. Like p27 Kip1, Ngn2 transcripts and protein are present in a subset of VZ/SVZ cells, and double labeling showed that p27 Kip1 and Ngn2 proteins are extensively coexpressed in these cells (Fig. 4A). In contrast with p27 Kip1, however, Ngn2 is sharply down-regulated as cells leave the germinal zones and migrate through the IZ (Fig. 4A, insets). To address the possibility that the two factors interact when coexpressed in VZ/SVZ cells, we examined the effect of manipulating p27 Kip1 expression on Ngn2 mrna and protein distribution. Overexpression of p27 wt or p27 ck resulted in a marked increase in number of electroporated cells expressing Ngn2 protein compared with control. Conversely, knockdown of p27 Kip1 expression led to a decrease in the number of Ngn2 + cells (Fig. 4B,C). Strikingly, the cortical VZ/SVZ of p27 Kip1 - null mutant embryos also showed a reduction in Ngn2 + cells, while p27 CK knock-in embryos have a normal level of Ngn2 expression compared to wild-type embryos (Fig. 4D). Up-regulation of Ngn2 expression was also observed when the N-terminal part of p27 ck (p27 ck N- term; see Materials and Methods) was overexpressed, while the C-terminal portion had no activity (p27 C- term; Fig. 4C). No change in distribution of Ngn2 transcripts was observed following electroporation of p27 wt, p27 ck,orp27 sirna, or in p27 Kip1 -null mutant embryos (not shown), suggesting that p27 Kip1 regulates Ngn2 at a post-transcriptional level. The sharp down-regulation of Ngn2 expression as newborn neurons leave the VZ/SVZ and migrate into the IZ (Fig. 4A; see also Kawaguchi et al. 2004; Hand et al. 2005; Britz et al. 2006) suggested that Ngn2 protein is rapidly turned over in these cells. One possible mecha- Figure 4. p27 Kip1 stabilizes Ngn2 protein in cortical progenitors. (A) Double immunostaining for p27 Kip1 (green) and Ngn2 (red) in a E15.5 mouse cortex showing coexpression of the two proteins in a subset of SVZ/VZ progenitors. Insets show enlargements of regions indicated in boxes. (B) Double immunostainings for electroporated cells overexpressing or down-regulating p27 Kip1 as indicated (GFP in green) and for endogenous Ngn2 (red). (C) Percentage of VZ cells expressing Ngn2 protein after electroporation of various forms of p27 Kip1 and sirna in E15.5 VZ progenitors and 24-h slice culture. Electroporation of p27 sirna#1 down-regulates Ngn2 protein and overexpression of p27 wt, p27 ck,or the N-terminal half of p27 ck (p27 ck N-term) up-regulate Ngn2 protein, while the C-terminal part (p27 C- term) has no activity (n = 3 5 slices; *p < 0.05, **p < 0.01). (D) Percentage of VZ cells expressing Ngn2 protein in E15.5 cortical progenitors from wild-type, p27 / -null mutant p27 ck embryos (n =3 6 brains; *p < 0.05). Panels showing immunostainings for Ngn2 (green) and TOTO-3 nuclear staining (red) illustrate the reduction in Ngn2 expression in absence of p27 Kip1 (p27 / ) and its restoration to wild-type levels by the cell cycle mutant allele p27 CK.(E) Ngn2 protein degradation analyzed by pulse-chase in rabbit reticulocytes (see Materials and Methods for details). Coexpression of Ngn2 with p27 wt and p27 ck markedly increased Ngn2 protein stability. In vitro translated p27 wt and p27 ck proteins were stable during the course of the experiment. Three experiments were performed and one representative example is shown GENES & DEVELOPMENT
7 Multiple functions of p27 in neurogenesis nism of post-transcriptional regulation could thus be at the level of Ngn2 protein stability. Several bhlh proteins with short half-lives are ubiquitinated and degraded by the 26S proteasome in an ATP-dependent fashion (Abu Hatoum et al. 1998; Sriuranpong et al. 2002). We measured the half-life of Ngn2 using an in vitro degradation assay in rabbit reticulocyte lysate, which is rich in multiple proteases including the 26S proteasome (Hoffman et al. 1992). In this assay, Ngn2 was rapidly degraded, with a half-life of 30 min, in an ATP-dependent manner, as degradation was blocked in the presence of the nonhydrolysable analog, ATP- S (not shown). When in vitro translated p27 wt or p27 ck was added to the assay, the half-life of Ngn2 was extended to 70 min or 100 min, respectively (Fig. 4E), while p21 wt had no effect (not shown). Thus, regulation by p27 Kip1 of Ngn2 expression in cortical VZ/SVZ likely involves stabilizing Ngn2 protein. This activity lies in the N-terminal part of p27 Kip1 and is independent of interaction with cyclin/cdk. p27 Kip1 promotes cortical neuron differentiation via regulation of Ngn2 The previous results raised the possibility that p27 Kip1 regulates the differentiation and/or migration of cortical neurons by regulating Ngn2 expression. To address this possibility, we tested the capacity of Ngn2 to rescue the differentiation and migration defects caused by p27 Kip1 knockdown. First, we established that Ngn2 regulates neuronal differentiation and radial migration in the cortical slice culture assay. In cortices isolated from Ngn2- null mutant embryos (Ngn2 null ), the fraction of cells expressing HuC/D 4 d after electroporation at E14.5 with a GFP vector was smaller than in wild-type cortices. Acute deletion of Ngn2 by electroporation of a Cre recombinase vector in the cortex of an embryo homozygous for a floxed allele of Ngn2 (Ngn2 floxed ) also resulted in a reduction in HuC/D expression. Conversely, overexpression of Ngn2 promoted HuC/D expression in electroporated cells (Fig. 5A). Ngn2 also regulated radial migration in this assay (see also Hand et al. 2005; Ge et al. 2006). A smaller fraction of cortical cells reached the CP after 4 d in Ngn2 null cortices or in Ngn2 floxed cortices electroporated with Cre than in wild-type cortices, while Ngn2 overexpression resulted in a small but significant increase in the number of cells reaching the CP (Fig. 5B). Thus, Ngn2 is involved in both neuronal differentiation and radial migration of cortical neurons in the slice culture assay. We next asked whether Ngn2 was mediating the cell Figure 5. Ngn2 overexpression rescues the neuronal differentiation defect but not the radial migration defect induced by p27 Kip1 knockdown. (A) Loss of Ngn2 function results in neuronal differentiation defects, as shown by reduced HuC/D expression in cortices of Ngn2 homozygous null mutant embryos (Ngn2 null ) electroporated with GFP at E14.5 and cultivated as slices for 4 d, and in cortices of embryos homozygous for a floxed allele of Ngn2 (Ngn2 floxed ) coelectroporated with Cre and GFP. Ngn2 overexpression conversely promotes neuronal differentiation, as shown by increased HuC/D expression in Ngn2 electroporated cells. The defect in HuC/D expression in cells electroporated with p27 sirna#1 is fully rescued by coelectroporation of Ngn2 (n = 5 13 slices; *p < 0.05, **p < 0.01). Panels showing double immunostainings for GFP (green) and HuC/D (red) illustrate the rescue by Ngn2 of the neuronal differentiation defect induced by p27 Kip1 knockdown. High magnification pictures show electroporated cortical neurons that have differentiated and remained in the VZ/SVZ compartment. (B) Loss of Ngn2 results in radial migration defects, as shown by electroporation of GFP in E14.5 Ngn2 null cortices or of Cre and GFP in E14.5 Ngn2 floxed cortices. Ngn2 overexpression results in a small but significant increase in the percentage of electroporated cells reaching the CP in wild-type cortex. However, Ngn2 overexpression does not rescue the migration defect resulting from knockdown of p27 Kip1 when coelectroporated with p27 sirna#1 (n =3 8 slices; *p < 0.05, **p < 0.01, **p < 0.001). Panels illustrate the migration phenotypes resulting from Ngn2 overexpression (Ngn2) and p27 Kip1 knockdown (p27 sirna#1) and the lack of rescue when Ngn2 is coelectroporated with p27 sirna#1. Electroporated cells are labeled for GFP. GENES & DEVELOPMENT 1517
8 Nguyen et al. cycle-independent functions of p27 Kip1 in cortical neurons. For this, we examined the capacity of Ngn2 to rescue p27 Kip1 knockdown phenotypes. Coexpression of Ngn2 with a p27 sirna resulted in a large number of cells expressing HuC/D, similar to that observed with Ngn2 alone and much higher than with p27 sirna alone or a control GFP vector (Fig. 5A). This demonstrates that overexpression of Ngn2 can rescue the neuronal differentiation defect produced by p27 Kip1 knockdown and suggests that regulation of Ngn2 is the main mechanism by which p27 Kip1 promotes neuronal differentiation. In striking contrast with the differentiation phenotype, the radial migration defect caused by p27 Kip1 knockdown was not rescued by overexpression of Ngn2. Only a few cells expressing p27 sirna reached the CP, and this was not significantly improved when Ngn2 was coexpressed with p27 sirna, although Ngn2 alone promoted migration to the CP (Fig. 5B). Therefore, p27 Kip1 promotes cortical neuron migration by a mechanism that does not involve Ngn2 and is therefore distinct from the mechanism underlying its differentiation activity. p27 Kip1 promotes cortical neuron migration by inhibiting RhoA/ROCK activity Cortical neuron migration has been shown to require inactivation of the GTPase RhoA (Kholmanskikh et al. 2003; Hand et al. 2005), which is expressed in the VZ/ SVZ (Fig. 6A). Coexpression of a dominant-negative version of RhoA (Wennerberg et al. 2003) with p27 sirna completely rescued neuronal migration to the CP (Fig. 6B). Cultivating cortical slices electroporated with p27 sirna#1 in the presence of 10 µm of CY27632, an inhibitor of the RhoA targets ROCK1 and ROCK2, also rescued migration to the CP (Fig. 6B). In contrast, coexpression of Rac1 with p27 sirna did not rescue the migration of neurons to the CP (Fig. 6B), demonstrating the specificity of dominant-negative RhoA in this assay. Together, these results demonstrate that inactivation of the RhoA/ROCK1/2 signaling pathway is an important mechanism by which p27 Kip1 regulates cortical neuron migration. The N- and C-terminal halves of p27 Kip1 have different cell cycle-independent activities The rescue experiments reported above suggested that p27 Kip1 independently regulates the differentiation and radial migration of cortical neurons. To directly address this possibility, we asked whether these two functions reside in different domains of the p27 Kip1 protein and could be physically dissociated, by separately testing the activities of the N-terminal and C-terminal halves of p27 Kip1. Expression of p27 ck N-term (N-terminal half of p27 Kip1 containing the cell cycle mutation) (Supplementary Fig. 1) induced expression of Ngn2 (Fig. 4C) and promoted HuC/D expression as efficiently as full-length p27 ck (Fig. 7A; Supplementary Fig. 4C) but did not promote migration to the CP (Fig. 7B). In contrast, p27 C- term (C-terminal half of p27 Kip1 ) (Supplementary Fig. 1) significantly promoted migration to the CP, less efficiently than full-length p27 ck but to a level similar to that of dominant-negative RhoA alone (Fig. 7B). p27 C- term also induced HuC/D expression, but significantly less efficiently than p27 ck N-term (Fig. 7A), and had no effect on Ngn2 expression (Fig. 4C). Together, these results demonstrate that p27 Kip1 independently promotes neuronal differentiation through a Ngn2 stabilizing activity residing in its N-terminal half (Figs. 4C, 7A) and radial migration, mostly through its C-terminal half (Fig. 7B), likely by direct inactivation of RhoA (Besson et al. 2004). Discussion We have addressed in this article the mechanisms that underlie the coordination of multiple cellular events during neurogenesis. We show that p27 Kip1 regulates neuronal differentiation and neuronal migration in the cerebral cortex independently of its cell cycle regulatory function. Moreover, we show that these activities involve different parts of the protein and distinct downstream pathways. We discuss below these different activities and the role of p27 Kip1 as a modular protein that coordinately regulates cell cycle exit, differentiation, and migration during neurogenesis. Figure 6. Blocking RhoA-ROCK signaling pathway rescues the radial migration defect induced by p27 Kip1 knockdown. (A) In situ hybridization of a coronal section through E14.5 telencephalon with a RhoA probe showing high levels of RhoA transcripts in VZ and SVZ cells. (B) Blocking RhoA activity by electroporating a dominant-negative form of RhoA (DNRhoA) or blocking ROCK1/2 activity by exposing cortical slices to the specific inhibitor CY27632 fully rescues the radial migration defect resulting from electroporation of p27 sirna#1. Overexpression of Rac1 does not rescue this phenotype (n =5 13 slices; *p < 0.05, **p < 0.01). Panels on the right illustrate the rescue of the radial migration defect induced by p27 sirna#1 when RhoA signaling is inhibited GENES & DEVELOPMENT
9 Multiple functions of p27 in neurogenesis Figure 7. The N- and C-terminal halves of p27 Kip1 harbor different activities. (A) The N-terminal half of p27 ck (p27 ck N- term) efficiently promotes expression of the neuronal marker HuC/D, while the C-terminal half (p27 C-term) only has a moderate differentiation activity. (B) p27 C-term promotes cell migration to the CP as efficiently as DNRhoA, while p27 ck N-term has no migration activity. (C) Summary scheme illustrating the modular nature of the p27 Kip1 molecule and its role in coordinating multiple cellular pathways during neurogenesis. p27 Kip1 N-term harbors the cell cycle regulation function and the neuronal differentiation promoting activity, involving stabilization of Ngn2, while p27 Kip1 C-term harbors the radial migration promoting activity, involving inactivation of RhoA. p27 Kip1 and Ngn2 form a regulatory loop that coordinates cell cycle exit with differentiation and radial migration of cortical neurons. Black arrows represent nontranscriptional interactions, and white arrows represent transcriptional interactions. Regulation of neuronal differentiation We show here that p27 Kip1 promotes the neuronal differentiation of cortical progenitors and that this activity is mediated in large part by stabilization of Neurogenin2 protein. Overexpression of wild-type or a cell cycle mutant version of p27 Kip1 induced prematurely the neuronal differentiation markers III-tubulin and HuC/D in VZ/ SVZ cells. Reciprocally, our analysis of p27 Kip1 -null mutant cortices revealed a defect in expression of these markers by VZ/SVZ cells, and a similar phenotype was observed following p27 Kip1 down-regulation by sirna electroporation. Coexpression of Ngn2 with a p27 sirna more than compensated this defect and neuronal markers were up-regulated to the same level as with Ngn2 alone, demonstrating that Ngn2 acts genetically downstream of p27 Kip1 and that p27 Kip1 regulates neuronal differentiation primarily through regulation of Ngn2 expression. Indeed, overexpression and knockdown experiments showed that p27 Kip1 regulates Ngn2 expression in cortical progenitors and acts primarily by stabilizing Ngn2 protein. Xenopus p27 Xic1 has been shown to stabilize the Neurogenin protein X-NGNR1 (Vernon et al. 2003), indicating that p27 Kip1 has an evolutionary conserved role in coupling neuronal differentiation with cell cycle exit by independently regulating cyclin/cdk activity and Neurogenin protein stability. We can envisage different mechanisms by which p27 Kip1 stabilizes Neurogenin2. p27 Kip1 has been shown to interact with F-box proteins, the substrate-specific components of E3 ubiquitin ligases (Laman et al. 2005). It may thus directly interfere with the activity of an E3 ubiquitin ligase that targets Ngn2 for degradation. Alternatively, p27 Kip1 might bind Ngn2 and protect it from degradation, as demonstrated for the stabilization of MyoD by p57 Kip2 (Reynaud et al. 2000). Besides stabilizing Ngn2, p27 Kip1 may also interact with other factors involved in neuronal differentiation. Indeed, regulation of white blood cell differentiation by p21 Cip1 involves interaction with multiple regulatory proteins, including the transcription factors Stat3 and C/EBP and the cofactor p300 (Steinman 2002). Is stability of Ngn2 a limiting factor for the differentiation of VZ/SVZ cells? Genetic experiments have established that Ngn2 activates a neuronal differentiation program when expressed in neural progenitors (Bertrand et al. 2002). Detailed studies of Ngn2 protein distribution in the cortex have shown that Ngn2 protein expression is induced in VZ progenitors after their last mitosis, that it is maintained in SVZ cells and then down-regulated in newborn neurons as they migrate through the IZ (Kawaguchi et al. 2004; Hand et al. 2005; Britz et al. 2006). Stabilization of Ngn2 by p27 Kip1 could result in accelerated accumulation of Ngn2 protein in VZ cells and/or in delayed downregulation of Ngn2 in the SVZ or IZ. Whether p27 Kip1 activity depends mostly on accelerating the accumulation or delaying the degradation of Ngn2 depends on when exactly Ngn2 activates the downstream differentiation program, which is not known. Ngn2 is thought to promote neuronal differentiation by activating a cascade of transcriptional regulators, including bhlh genes such as NeuroD1, Math3/NeuroM, and Math2/Nex, and T-box genes such as Tbr2 and Tbr1 (Bertrand et al. 2002; Schuurmans et al. 2004; Englund et al. 2005). However, which among these genes are direct targets of Ngn2 and what is their precise timing of expression in cortical neurons remains to be determined. Regulation of radial migration p27 Kip1 has been shown to regulate the migration of different cell types in vitro, but its role in cell migration in vivo, let alone in the cortex, has not yet been established. We have demonstrated that p27 Kip1 is an important player in the control of cortical neuron migration. We show for the first time that p27 Kip1 -deficient embryos present defects in the radial migration of cortical neurons. This phenotype is not due to defects in cell cycle exit since the cortex of a mouse homozygous for the knock-in cell cycle mutant allele p27 CK (Besson et al. 2006) is not affected. No defects related to abnormal cortical neuronal migration have previously been reported in adult p27 Kip1 -deficient mice. Whether the reduced mi- GENES & DEVELOPMENT 1519
10 Nguyen et al. gration that we observed at E17.5 is a transient defect that is eventually compensated remains to be addressed. Supporting an important role of p27 Kip1 in migration, overexpression of the wild-type or the cell cycle mutant versions of p27 Kip1 efficiently promoted the migration of cortical neurons to the cortical plate, while p27 Kip1 down-regulation largely inhibited this migration. Since Ngn2 has also been shown recently to regulate cortical neuron migration (Hand et al. 2005), we anticipated that Ngn2 would mediate both the differentiation and migration activities of p27 Kip1. However, coexpression of Ngn2 and a p27 sirna did not ameliorate the migration defect caused by p27 Kip1 knockdown, indicating that the migration activity of p27 Kip1 involves a different pathway. In support of this idea, p27 Kip1 migration activity resides entirely in its C-terminal portion, whereas its differentiation activity resides mostly in its N-terminal part. Regulation of fibroblast migration by p27 Kip1 involves inactivation of the small GTPase RhoA (Besson et al. 2004). We thus examined whether the same mechanism was operating in the cortex and found that indeed expression of a dominant-negative form of RhoA (DNRhoA) or exposure to a pharmacological inhibitor of the RhoA effectors ROCK1/2 efficiently rescued the migration defect resulting from p27 Kip1 knockdown. Regulation of cell migration by inhibition of RhoA activity is therefore likely to be a general function of p27 Kip1 in various tissues. A recent study has identified the actinbinding protein cofilin as a phosphorylation substrate of RhoA and proposed that derepression of cofilin underlies p27 Kip1 activity in cortical neuron migration (Kawauchi et al. 2006). The lack of rescue of the p27 Kip1 migration phenotype by Ngn2 may come as a surprise since RhoA down-regulation has also been proposed as a mechanism by which Ngn2 promotes cortical neuron migration, as shown by repression of RhoA transcription by Ngn2 (Ge et al. 2006) and by the rescue of migration defects in Ngn2 mutant cortex by DNRhoA (Hand et al. 2005). However, the repression of RhoA by Ngn2 was not very robust, and the rescue of the Ngn2 mutant migration defect by DNRhoA was only partial. Therefore, downregulation of RhoA is unlikely to be the main mechanism whereby Ngn2 promotes cortical neuron migration, and Ngn2 has indeed been shown to regulate other migration genes such as Dcx and p35 (Ge et al. 2006). This likely explains why Ngn2 overexpression does not repress RhoA to a sufficient extent to rescue the p27 Kip1 migration phenotype. The complete rescue of this defect by DNRhoA indicates that inactivation of RhoA/ ROCK1/2 signaling is the major mechanism by which p27 Kip1 promotes cortical neuron migration. However, since p27 Kip1 also regulates Ngn2, which, in turn, regulates multiple migration genes (Ge et al. 2006; our unpublished data), p27 Kip1 is likely to activate cortical neuron migration via multiple pathways (Fig. 7C). p27 Kip1 as a modular protein coupling multiple pathways The tight coupling of multiple cellular processes is a striking feature of the neurogenesis program. Although progress has been made in characterizing the molecular pathways that regulate individual cellular events during neurogenesis, including cell cycle control (Ohnuma and Harris 2003), neuronal differentiation (Guillemot et al. 2006), and particularly neuronal migration (Bielas et al. 2004), how these various processes are coordinately regulated in a coherent developmental program remains poorly understood. Here, we provide evidence that p27 Kip1 plays an important role in the coordination of multiple aspects of neurogenesis in the cortex. The role of p27 Kip1 in controlling the timing of cell cycle exit of cortical progenitors is well established (Goto et al. 2004; Lukaszewicz et al. 2005; Tarui et al. 2005). We show in this article that p27 Kip1 also promotes the differentiation and migration of newborn cortical neurons, independently of this cell cycle function. Importantly, the multifunctionality of p27 Kip1 relies on the existence of different domains in the molecule that independently regulate distinct pathways. The C-terminal half of p27 Kip1, which has been shown to directly interact with RhoA and inhibit its activation (Besson et al. 2004), harbors the migration-promoting activity of p27 Kip1 and functions in isolation from the N-terminal half, although not as well as the full-length molecule. The N-terminal half, which also contains the cyclin/cdk-interacting domain of p27 Kip1, promotes Ngn2 protein expression and neuronal differentiation as efficiently as wild-type p27 Kip1. Although the C-terminal half does not influence Ngn2 expression, it has a moderate differentiation-promoting activity on its own, suggesting the existence of additional pathways regulating neuronal differentiation downstream of p27 Kip1. Interestingly, p27 Kip1 protein is present in both cytoplasm and nucleus, in different ratios at different stages of differentiation (Fig. 1), and it likely regulates different processes in different cellular compartments, interacting with cyclin/cdks and stabilizating Ngn2 in the nucleus while interacting with RhoA in the cytoplasm. Moreover, the different activities of p27 Kip1 may be segregated not only spatially but also temporally. The regulation of cell cycle exit and the promotion of Ngn2 stability and differentiation take place in the VZ/SVZ before the last division of progenitors and during the following G1 phase, while its migration promoting activity may take place in the IZ, when neurons are motile. p27 Kip1 expression remains high in neurons that have reached their final position in the cortical plate, suggesting that p27 Kip1 may yet have additional functions at later stages. Besides p27 Kip1, Ngn2 also plays an important role in coordinating neurogenesis in the cortex, by independently regulating neuronal differentiation, specification of multiple aspects of cortical neuron identity, and neuronal migration (Bertrand et al. 2002; Schuurmans et al. 2004; Hand et al. 2005). Experiments in cultured cells have provided evidence that neural bhlh genes including Neurogenins induce expression of p27 Kip1 and promote cell cycle exit (Farah et al. 2000), and we have shown that p27 Kip1 regulates Ngn2 expression in cortical progenitors, suggesting that these two molecules are engaged in a regulatory loop. Thus, a regulatory network 1520 GENES & DEVELOPMENT
11 Multiple functions of p27 in neurogenesis involving p27 Kip1, Ngn2, and perhaps other multifunctional proteins (Ferguson et al. 2005) coordinates the various pathways that contribute to the neurogenic program in the cortex. Materials and methods Animals Mice were housed, bred, and treated according to the guidelines approved by the Home Office under the Animals (Scientific procedures) Act The generation and genotyping of transgenic mice have been reported previously: Ngn2 KILacZ (Nieto et al. 2001), Ngn2 KIloxNgn2 (Hand et al. 2005), p27 CK (Besson et al. 2006), and p27 (Fero et al. 1996). RNA in situ hybridization and immunohistochemistry Embryonic brains were dissected in 1 phosphate buffered saline (PBS) and fixed for 45 min (for immunohistochemistry) or overnight (for RNA in situ hybridization) in 4% paraformaldehyde (PFA)/1 PBS at 4 C. Cultivated brain slices were fixed in 4% PFA/1 PBS for 30 min. Fixed samples were cryoprotected overnight in 20% sucrose/1 PBS at 4 C and mounted in OCT Compound (VWR) and sectioned coronally (10 µm) with a cryostat (CM3050S, Leica). Nonradioactive RNA in situ hybridization was performed as described previously (Cau et al. 1997) using antisense RNA probes for p21 Cip1 (gift from Dr. Jay Cross, University of Calgary, Alberta, Canada), p27 Kip1 and p57 Kip2 (gift from Dr. A. Mallamaci, San Raffaelle Scientific Institute, Milano, Italy), RhoA (gift from Dr. Y.E. Sun, University of California at Los Angeles, CA), and Ngn2 (Gradwohl et al. 1996). For immunohistochemistry, cryostat sections were washed three times in PBST (PBS, 0.1% Triton X-100), and blocked at room temperature for 1hinPBST supplemented with 10% goat serum (Vector Laboratories). Primary antibodies were incubated overnight at 4 C; rat anti-brdu (Oxford Biotechnology; 1:20), rabbit anti- BLBP (gift from Dr. M. Götz, Forschungszentrum für Umwelt und Gesundheit, Neuherberg, Germany; 1:1000), rabbit anti- GFP (Molecular Probes; 1:2000), chicken anti-gfp (Chemicon; 1:500), mouse anti-nestin (Rat 401, Developmental Hybridoma Bank; 1:10), mouse anti-ngn2 (1:20, gift from Dr. David Anderson, California Institute of Technology, Pasadena, CA), mouse anti- III-tubulin (Tuj1, Covance; 1:1000), mouse anti-huc/d (Molecular Probes; 1:100), rabbit anti-p21 Cip1 (C-19, Santa Cruz Biotechnology; 1:400), mouse anti-p27 Kip1 (BD Biosciences; 1:200), rabbit anti-p27 Kip1 N terminus (N-20, Santa Cruz Biotechnology; 1:100), rabbit anti-p27 Kip1 C terminus (Lab vision; 1:100), rabbit anti-p57 Kip2 (C-20, Santa Cruz Biotechnology; 1:100), and rabbit anti-activated caspase-3 (R&D system; 1:1000). Slides for BrdU detection were pretreated with 2N HCl for 30 min. After washing, slides were incubated for 1 h at room temperature with the appropriate secondary antibodies: goat anti-mouse Alexa Fluor 488, goat anti-mouse Alexa Fluor 568, goat anti-chicken Alexa Fluor 488, goat anti-rabbit Alexa Fluor 488, goat anti-rabbit Alexa Fluor 568 (Molecular Probes; 1:1000), and Cy5 conjugated goat anti-rat (Jackson Immunoresearch; 1:500). For in vivo BrdU incorporation, pregnant females were injected intraperitoneally at E14.5 with 100 mg BrdU/kg body mass (Boehringer Mannheim #280879) and embryos were harvested at E17.5. For counting, cells were counterstained with TOTO-3 (Molecular Probes; 1:1000) during secondary antibody incubation. Sections were washed three times in PBST and coverslipped using Aqua Polymount (Polysciences, Inc.). Images were acquired using a laser scanning confocal microscope (Radiance 2100, Bio-Rad). Plasmids and sirna Plasmid DNA were prepared using a Plasmid Maxi Kit (Qiagen). pcdna-rhoat19n and pcdna-rac1 were purchased from University of Missouri-Rolla cdna Resource Center; pcs2- p21 wt and pcs2-p21 N50S were gifts from S. Ohnuma (University of Cambridge, UK); pqcxipp27 wt, pcdnap27 wt, pcdnap27 ck, and pcdna-p27c-term were constructed by A. Besson (Besson et al. 2004); pcdna-ha-p57 wt and pcdna- HA-p57 ck were provided by Y. Xiong (University of North Carolina at Chapel Hill, NC) (Watanabe et al. 1998); and p27 ck N-term cdna (amino acids 1 86) was generated by PCR using pcdna3.1 p27 ck (Besson et al. 2004). All full-length cdna were subcloned into a pcaggs-ires-gfp vector generously provided by J. Briscoe (National Institute for Medical Research, London, UK), where a [cdna]-nlsires-egfp cassette is under the control of an CMV-enhancer and a chicken -actin promoter. All constructs were verified by sequencing. The following sirnas were purchased from Ambion: 5 -GCU UGCCCGAGUUCUACUAtt-3 (sense strand) and 5 -UAG UAGAACUCGGGCAAGCtg-3 (antisense strand) for p27 Kip1 sirna#1 (predesigned sirna no directed against the exon 1 of mouse and human p27 Kip1 ); 5 -GGUAUUUUUCAAG AUUACGtt-3 (sense strand) and 5 -CGUAAUCUUGAAAA AUACCtg-3 (antisense strand) for p27 Kip1 sirna#2 (predesigned sirna no directed against the exon 2 of mouse p27 Kip1 ). The extent of p27 Kip1 knockdown elicited by both sirna was compared with that of a control sirna (Silencer Negative Control#1 sirna no. 4611) with no significant homology with known gene sequences in rodent and human. In utero electroporation, ex vivo electroporation, and dissociated cell culture In utero electroporation was performed as described previously (Saito and Nakatsuji 2001) with minor modifications (see Supplemental Material). Ex vivo electroporation was performed on injected mouse embryos heads by using electrical settings similar to those applied for in vivo electroporation (see Supplemental Material). Following electroporation, brains were dissected in L15 (Invitrogen) and transferred into liquid 3% low melting agarose (38 C; Sigma) and incubated on ice for 1 h. Embedded brains were cut coronally (250 µm) with a vibratome (VT1000S, Leica), and slices were transferred onto sterilized culture plate inserts (0.4-µm pore size; Millicell-CM, Millipore) and cultivated in semidry condition in wells containing Neurobasal medium supplemented with B27 (1%), N2 (1%), glutamine (1%), penicillin/streptomycin (1%), fungizone (0.1%; Invitrogen), and ciprofloxacine (5 µg/ml; MP Biomedicals). Slices were cultivated for up to 4 d, with half the culture medium renewed every day. For identification of cells cycling at the time of electroporation, slices were incubated with 10 µm BrdU (Sigma) until the end of the culture. For dissociated cell cultures, slices were cultivated 2 d following electroporation, the VZ/SVZ was microdissected under a GFP binocular to identify the electroporated regions, and tissues were pooled and incubated with 0.05% trypsin (Invitrogen) for 15 min followed by trituration in DMEM FCS 10% with firepolished Pasteur pipettes. Fifty microliters of dissociated cells in suspension ( /ml) were seeded for 30 min on poly-dlysine-coated coverslips in 24-well plates, then covered with GENES & DEVELOPMENT 1521
(a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for
 Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
Supplementary Figure S1. Alterations in Fzr1( / );Nestin-Cre brains. (a) P10 Cdh1-deficient brains display low levels of Myelin basic protein (MBP)
 Supplementary Figure S1. Alterations in Fzr1( / );Nestin-Cre brains. (a) P10 Cdh1-deficient brains display low levels of Myelin basic protein (MBP) in the cortex (area 1 as defined in Fig. 2a), and corpus
Supplementary Figure S1. Alterations in Fzr1( / );Nestin-Cre brains. (a) P10 Cdh1-deficient brains display low levels of Myelin basic protein (MBP) in the cortex (area 1 as defined in Fig. 2a), and corpus
Supplementary Material
 Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl
 Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Supplemental Information
 Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
A population of Nestin expressing progenitors in the cerebellum exhibits increased tumorigenicity
 A population of Nestin expressing progenitors in the cerebellum exhibits increased tumorigenicity Peng Li 1,2, Fang Du 1, Larra W. Yuelling 1, Tiffany Lin 3, Renata E. Muradimova 1, Rossella Tricarico
A population of Nestin expressing progenitors in the cerebellum exhibits increased tumorigenicity Peng Li 1,2, Fang Du 1, Larra W. Yuelling 1, Tiffany Lin 3, Renata E. Muradimova 1, Rossella Tricarico
GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts
 Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Mouse Models to Investigate Cell Cycle and Cancer Prof. Philipp Kaldis
 Mouse Models to Investigate Institute of Molecular and Cell Biology (IMCB) Cell Division and Cancer Laboratory, Proteos 3-09 Singapore 138673 1 2 Crystal structure of Cdk2/ cyclin A A.A Russo et al., (1996)
Mouse Models to Investigate Institute of Molecular and Cell Biology (IMCB) Cell Division and Cancer Laboratory, Proteos 3-09 Singapore 138673 1 2 Crystal structure of Cdk2/ cyclin A A.A Russo et al., (1996)
Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and
 Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
Supplementary Figure 1 Phosphorylated tau accumulates in Nrf2 (-/-) mice. Hippocampal tissues obtained from Nrf2 (-/-) (10 months old, 4 male; 2
 Supplementary Figure 1 Phosphorylated tau accumulates in Nrf2 (-/-) mice. Hippocampal tissues obtained from Nrf2 (-/-) (10 months old, 4 male; 2 female) or wild-type (5 months old, 1 male; 11 months old,
Supplementary Figure 1 Phosphorylated tau accumulates in Nrf2 (-/-) mice. Hippocampal tissues obtained from Nrf2 (-/-) (10 months old, 4 male; 2 female) or wild-type (5 months old, 1 male; 11 months old,
DRG Pituitary Cerebral Cortex
 Liver Spinal cord Pons Atg5 -/- Atg5 +/+ DRG Pituitary Cerebral Cortex WT KO Supplementary Figure S1 Ubiquitin-positive IBs accumulate in Atg5 -/- tissues. Atg5 -/- neonatal tissues were fixed and decalcified.
Liver Spinal cord Pons Atg5 -/- Atg5 +/+ DRG Pituitary Cerebral Cortex WT KO Supplementary Figure S1 Ubiquitin-positive IBs accumulate in Atg5 -/- tissues. Atg5 -/- neonatal tissues were fixed and decalcified.
Supporting Information
 Supporting Information Ho et al. 10.1073/pnas.0808899106 SI Materials and Methods In immunostaining, antibodies from Developmental Studies Hybridoma ank were -FasII (mouse, 1:200), - (mouse, 1:100), and
Supporting Information Ho et al. 10.1073/pnas.0808899106 SI Materials and Methods In immunostaining, antibodies from Developmental Studies Hybridoma ank were -FasII (mouse, 1:200), - (mouse, 1:100), and
Flow cytometry Stained cells were analyzed and sorted by SORP FACS Aria (BD Biosciences).
 Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or
 Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Supplemental Figures Supplemental Figure 1
 Supplemental Figures Supplemental Figure 1 The de novo and salvage purine synthesis pathways are shown converging on GMP synthesis (A). Enzymes and intermediates not involved in MPA action and/or guanosine
Supplemental Figures Supplemental Figure 1 The de novo and salvage purine synthesis pathways are shown converging on GMP synthesis (A). Enzymes and intermediates not involved in MPA action and/or guanosine
Supplemental Information
 Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplemental Figure 1 (Figure S1), related to Figure 1 Figure S1 provides evidence to demonstrate Nfatc1Cre is a mouse line that directed gene
 Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
Schematic representation of the endogenous PALB2 locus and gene-disruption constructs
 Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Experimental genetics - 2 Partha Roy
 Partha Roy Experimental genetics - 2 Making genetically altered animal 1) Gene knock-out k from: a) the entire animal b) selected cell-type/ tissue c) selected cell-type/tissue at certain time 2) Transgenic
Partha Roy Experimental genetics - 2 Making genetically altered animal 1) Gene knock-out k from: a) the entire animal b) selected cell-type/ tissue c) selected cell-type/tissue at certain time 2) Transgenic
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Supplementary Methods
 Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
Supporting Information
 Supporting Information Lukaszewicz and Anderson 1.173/pnas.1162318 SI Text 1 Modeling GOF Electroporation. We tested whether mke overexpression could lead indirectly to an increase in the size of the neurogenic
Supporting Information Lukaszewicz and Anderson 1.173/pnas.1162318 SI Text 1 Modeling GOF Electroporation. We tested whether mke overexpression could lead indirectly to an increase in the size of the neurogenic
Flag-Rac Vector V12 V12 N17 C40. Vector C40 pakt (T308) Akt1. Myc-DN-PAK1 (N-SP)
 a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
Nature Medicine doi: /nm.2548
 Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
Learning Objectives. Define RNA interference. Define basic terminology. Describe molecular mechanism. Define VSP and relevance
 Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
Mechanisms of embryonic stem cell division and differentiation
 Mechanisms of embryonic stem cell division and differentiation Josephine Wbite.'.... Department of Molecular Biosciences (Biochemistry) The University of Adelaide Adelaide, South Australia Submitted for
Mechanisms of embryonic stem cell division and differentiation Josephine Wbite.'.... Department of Molecular Biosciences (Biochemistry) The University of Adelaide Adelaide, South Australia Submitted for
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11094 Supplementary Figures Supplementary Figure 1: Analysis of GFP expression in P0 wild type and mutant ( E1-4) Fezf2-Gfp BAC transgenic mice. Epifluorescent images of sagittal forebrain
doi:10.1038/nature11094 Supplementary Figures Supplementary Figure 1: Analysis of GFP expression in P0 wild type and mutant ( E1-4) Fezf2-Gfp BAC transgenic mice. Epifluorescent images of sagittal forebrain
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/310/5753/1487/dc1 Supporting Online Material for Stem Cell Self-Renewal Controlled by Chromatin Remodeling Factors Rongwen Xi and Ting Xie* *To whom correspondence should
www.sciencemag.org/cgi/content/full/310/5753/1487/dc1 Supporting Online Material for Stem Cell Self-Renewal Controlled by Chromatin Remodeling Factors Rongwen Xi and Ting Xie* *To whom correspondence should
SUPPLEMENTARY NOTE 3. Supplememtary Note 3, Wehr et al., Monitoring Regulated Protein-Protein Interactions Using Split-TEV 1
 SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES
 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Generation of inducible BICD2 knock-out mice. A) The mouse BICD2 locus and gene targeting constructs. To generate an inducible Bicd2
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Generation of inducible BICD2 knock-out mice. A) The mouse BICD2 locus and gene targeting constructs. To generate an inducible Bicd2
DCLK-immunopositive. Bars, 100 µm for B, 50 µm for C.
 Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supporting Information
 Supporting Information Nowakowski et al. 10.1073/pnas.1219385110 SI Materials and Methods Primary Antibodies. Primary antibodies used in this study were raised against BrdU (rat, 1:50; Abcam), cleaved
Supporting Information Nowakowski et al. 10.1073/pnas.1219385110 SI Materials and Methods Primary Antibodies. Primary antibodies used in this study were raised against BrdU (rat, 1:50; Abcam), cleaved
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various
 Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat
 Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat pups at P6, P14, P22, P30 and adult (A) rats were subjected
Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat pups at P6, P14, P22, P30 and adult (A) rats were subjected
Pei et al. Supplementary Figure S1
 Pei et al. Supplementary Figure S1 C H-CUL9: + + + + + Myc-ROC1: - - + + + U2OS/pcDN3 U2OS/H-CUL9 U2OS/ + H-CUL9 IP: -H -myc input -H -myc 1 2 3 4 5 H-CUL9 Myc-ROC1 -H -H -H -H H-CUL9: wt RR myc-roc1:
Pei et al. Supplementary Figure S1 C H-CUL9: + + + + + Myc-ROC1: - - + + + U2OS/pcDN3 U2OS/H-CUL9 U2OS/ + H-CUL9 IP: -H -myc input -H -myc 1 2 3 4 5 H-CUL9 Myc-ROC1 -H -H -H -H H-CUL9: wt RR myc-roc1:
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
embryos. Asterisk represents loss of or reduced expression. Brackets represent
 Supplemental Figures Supplemental Figure 1. tfec expression is highly enriched in tail endothelial cells (A- B) ISH of tfec at 15 and 16hpf in WT embryos. (C- D) ISH of tfec at 36 and 38hpf in WT embryos.
Supplemental Figures Supplemental Figure 1. tfec expression is highly enriched in tail endothelial cells (A- B) ISH of tfec at 15 and 16hpf in WT embryos. (C- D) ISH of tfec at 36 and 38hpf in WT embryos.
Nature Structural & Molecular Biology: doi: /nsmb.1583
 Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
TISSUE-SPECIFIC STEM CELLS
 TISSUE-SPECIFIC STEM CELLS Geminin Regulates Cortical Progenitor Proliferation and Differentiation MAGDA SPELLA, a CHRISTINA KYROUSI, a EVA KRITIKOU, a ATHANASIA STATHOPOULOU, a FRANÇOIS GUILLEMOT, b DIMITRIS
TISSUE-SPECIFIC STEM CELLS Geminin Regulates Cortical Progenitor Proliferation and Differentiation MAGDA SPELLA, a CHRISTINA KYROUSI, a EVA KRITIKOU, a ATHANASIA STATHOPOULOU, a FRANÇOIS GUILLEMOT, b DIMITRIS
Test Bank for Molecular Cell Biology 7th Edition by Lodish
 Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table.
 Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Nature Medicine doi: /nm.2558
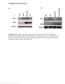 Supplementary. Fig. 1. (a) Sirt1 and mutant HTT (detected by HTT 81-90 antibody) protein levels were detected by Western blotting in cerebral cortex of N171-82Q mice. (b) Sirt1 and mutant HTT (detected
Supplementary. Fig. 1. (a) Sirt1 and mutant HTT (detected by HTT 81-90 antibody) protein levels were detected by Western blotting in cerebral cortex of N171-82Q mice. (b) Sirt1 and mutant HTT (detected
Loss of Cul3 in Primary Fibroblasts
 PSU McNair Scholars Online Journal Volume 5 Issue 1 Humans Being: People, Places, Perspectives and Processes Article 15 2011 Loss of Cul3 in Primary Fibroblasts Paula Hanna Portland State University Let
PSU McNair Scholars Online Journal Volume 5 Issue 1 Humans Being: People, Places, Perspectives and Processes Article 15 2011 Loss of Cul3 in Primary Fibroblasts Paula Hanna Portland State University Let
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
V4: Protein phosphorylation during cell cycle
 V4: Protein phosphorylation during cell cycle Protein phosphorylation and dephosphorylation are highly controlled biochemical processes that respond to various intracellular and extracellular stimuli.
V4: Protein phosphorylation during cell cycle Protein phosphorylation and dephosphorylation are highly controlled biochemical processes that respond to various intracellular and extracellular stimuli.
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and
 Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of
 Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
V6: Protein phosphorylation during cell cycle
 V6: Protein phosphorylation during cell cycle Protein phosphorylation and dephosphorylation are highly controlled biochemical processes that respond to various intracellular and extracellular stimuli.
V6: Protein phosphorylation during cell cycle Protein phosphorylation and dephosphorylation are highly controlled biochemical processes that respond to various intracellular and extracellular stimuli.
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
Supplemental Materials and Methods
 Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Methods Western blot analysis of plg Quantification of plasminogen accumulation by ELISA Immunohistochemical analysis
 Methods Western blot analysis of plg Wild-type mice first received a standardized burn wound and then were intravenously administered 2 mg of human plg (Omnio AB, Umeå, Sweden). 24 hours after wounding
Methods Western blot analysis of plg Wild-type mice first received a standardized burn wound and then were intravenously administered 2 mg of human plg (Omnio AB, Umeå, Sweden). 24 hours after wounding
Charles Shuler, Ph.D. University of Southern California Los Angeles, California Approved for Public Release; Distribution Unlimited
 AD Award Number: DAMD17-01-1-0100 TITLE: Smad-Mediated Signaling During Prostate Growth and Development PRINCIPAL INVESTIGATOR: Charles Shuler, Ph.D. CONTRACTING ORGANIZATION: University of Southern California
AD Award Number: DAMD17-01-1-0100 TITLE: Smad-Mediated Signaling During Prostate Growth and Development PRINCIPAL INVESTIGATOR: Charles Shuler, Ph.D. CONTRACTING ORGANIZATION: University of Southern California
- Supplementary Information -
 TRIM32 dependent transcription in adult neural progenitor cells regulates neuronal differentiation. - Supplementary Information - Anna-Lena Hillje 1, 2#, Maria Angeliki S. Pavlou 1, 2#, Elisabeth Beckmann
TRIM32 dependent transcription in adult neural progenitor cells regulates neuronal differentiation. - Supplementary Information - Anna-Lena Hillje 1, 2#, Maria Angeliki S. Pavlou 1, 2#, Elisabeth Beckmann
Supplementary Fig. 1. Schematic structure of TRAIP and RAP80. The prey line below TRAIP indicates bait and the two lines above RAP80 highlight the
 Supplementary Fig. 1. Schematic structure of TRAIP and RAP80. The prey line below TRAIP indicates bait and the two lines above RAP80 highlight the prey clones identified in the yeast two hybrid screen.
Supplementary Fig. 1. Schematic structure of TRAIP and RAP80. The prey line below TRAIP indicates bait and the two lines above RAP80 highlight the prey clones identified in the yeast two hybrid screen.
Supplementary Fig. 1. (A) Working model. The pluripotency transcription factor OCT4
 SUPPLEMENTARY FIGURE LEGENDS Supplementary Fig. 1. (A) Working model. The pluripotency transcription factor OCT4 directly up-regulates the expression of NIPP1 and CCNF that together inhibit protein phosphatase
SUPPLEMENTARY FIGURE LEGENDS Supplementary Fig. 1. (A) Working model. The pluripotency transcription factor OCT4 directly up-regulates the expression of NIPP1 and CCNF that together inhibit protein phosphatase
Figure S1. Related to Figure 1, to show 3-D images of nerve/vessel patterning.
 Supplemental Information Inventory of Supplemental Information Figure S1. Related to Figure 1, to show 3-D images of nerve/vessel patterning. Figure S2. Related to Figure 4, to validate Npn-1 signaling
Supplemental Information Inventory of Supplemental Information Figure S1. Related to Figure 1, to show 3-D images of nerve/vessel patterning. Figure S2. Related to Figure 4, to validate Npn-1 signaling
Supplemental data. Supplemental Materials and Methods
 Supplemental data Supplemental Materials and Methods Transfection of plasmid. Transfection of plasmids into FRTL5 cells was performed using Lipofectamine LTX with Plus reagent (Invitrogen) according to
Supplemental data Supplemental Materials and Methods Transfection of plasmid. Transfection of plasmids into FRTL5 cells was performed using Lipofectamine LTX with Plus reagent (Invitrogen) according to
SUPPLEMENTARY INFORMATION
 VOLUME: 1 ARTICLE NUMBER: 0011 In the format provided by the authors and unedited. In situ Activation of Platelets with Checkpoint Inhibitors for Post-Surgical Cancer Immunotherapy Chao Wang 1, 2, Wujin
VOLUME: 1 ARTICLE NUMBER: 0011 In the format provided by the authors and unedited. In situ Activation of Platelets with Checkpoint Inhibitors for Post-Surgical Cancer Immunotherapy Chao Wang 1, 2, Wujin
Sperm cells are passive cargo of the pollen tube in plant fertilization
 In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17079 Sperm cells are passive cargo of the pollen tube in plant fertilization Jun Zhang 1, Qingpei
In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17079 Sperm cells are passive cargo of the pollen tube in plant fertilization Jun Zhang 1, Qingpei
In general, 8- to 10-week-old adult females were ovariectomized and rested for 10 days
 Animal injections and tissue processing In general, 8- to 10-week-old adult females were ovariectomized and rested for 10 days before they received any injections. Mice were injected with sesame seed oil
Animal injections and tissue processing In general, 8- to 10-week-old adult females were ovariectomized and rested for 10 days before they received any injections. Mice were injected with sesame seed oil
Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and
 SUPPLEMENTARY MATERIALS AND METHODS Chromatin Immunoprecipitation for qpcr analysis Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and IL24, all located on chromosome 1. Primer
SUPPLEMENTARY MATERIALS AND METHODS Chromatin Immunoprecipitation for qpcr analysis Four different active promoter genes were chosen, ATXN7L2, PSRC1, CELSR2 and IL24, all located on chromosome 1. Primer
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature22047 Supplementary discussion Overall developmental timeline and brain regionalization in human whole-brain organoids We first defined the timeline of generation of broadly-defined cell
doi:10.1038/nature22047 Supplementary discussion Overall developmental timeline and brain regionalization in human whole-brain organoids We first defined the timeline of generation of broadly-defined cell
Xu et al., Supplementary Figures 1-7
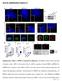 Xu et al., Supplementary Figures 1-7 Supplementary Figure 1. PIPKI is required for ciliogenesis. (a) PIPKI localizes at the basal body of primary cilium. RPE-1 cells treated with two sirnas targeting to
Xu et al., Supplementary Figures 1-7 Supplementary Figure 1. PIPKI is required for ciliogenesis. (a) PIPKI localizes at the basal body of primary cilium. RPE-1 cells treated with two sirnas targeting to
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Apoptosis analyses and quantification. Activated caspase3 immunostaining on sections of E9.0 Tcof1 +/ -;p53 +/- (a) and Tcof1 +/ -;p53 -/- (b) littermate embryos
Supplementary Figures Supplementary Figure 1. Apoptosis analyses and quantification. Activated caspase3 immunostaining on sections of E9.0 Tcof1 +/ -;p53 +/- (a) and Tcof1 +/ -;p53 -/- (b) littermate embryos
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/4/9/eaat5401/dc1 Supplementary Materials for GLK-IKKβ signaling induces dimerization and translocation of the AhR-RORγt complex in IL-17A induction and autoimmune
advances.sciencemag.org/cgi/content/full/4/9/eaat5401/dc1 Supplementary Materials for GLK-IKKβ signaling induces dimerization and translocation of the AhR-RORγt complex in IL-17A induction and autoimmune
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling
 Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary Figure Legends
 Supplementary Figure Legends Figure S1 gene targeting strategy for disruption of chicken gene, related to Figure 1 (f)-(i). (a) The locus and the targeting constructs showing HpaI restriction sites. The
Supplementary Figure Legends Figure S1 gene targeting strategy for disruption of chicken gene, related to Figure 1 (f)-(i). (a) The locus and the targeting constructs showing HpaI restriction sites. The
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
Supplemental Data. Wu et al. (2). Plant Cell..5/tpc RGLG Hormonal treatment H2O B RGLG µm ABA µm ACC µm GA Time (hours) µm µm MJ µm IA
 Supplemental Data. Wu et al. (2). Plant Cell..5/tpc..4. A B Supplemental Figure. Immunoblot analysis verifies the expression of the AD-PP2C and BD-RGLG proteins in the Y2H assay. Total proteins were extracted
Supplemental Data. Wu et al. (2). Plant Cell..5/tpc..4. A B Supplemental Figure. Immunoblot analysis verifies the expression of the AD-PP2C and BD-RGLG proteins in the Y2H assay. Total proteins were extracted
Figure S2. Response of mouse ES cells to GSK3 inhibition. Mentioned in discussion
 Stem Cell Reports, Volume 1 Supplemental Information Robust Self-Renewal of Rat Embryonic Stem Cells Requires Fine-Tuning of Glycogen Synthase Kinase-3 Inhibition Yaoyao Chen, Kathryn Blair, and Austin
Stem Cell Reports, Volume 1 Supplemental Information Robust Self-Renewal of Rat Embryonic Stem Cells Requires Fine-Tuning of Glycogen Synthase Kinase-3 Inhibition Yaoyao Chen, Kathryn Blair, and Austin
Supplementary Figure 1. TSA (10 nmol/l), non-class-selective HDAC inhibitor, potentiates
 Supplementary Figure 1. TSA (10 nmol/l), non-class-selective HDAC inhibitor, potentiates vascular calcification (VC). (a) Von Kossa staining shows that TSA potentiated the Pi-induced VC. Scale bar, 100
Supplementary Figure 1. TSA (10 nmol/l), non-class-selective HDAC inhibitor, potentiates vascular calcification (VC). (a) Von Kossa staining shows that TSA potentiated the Pi-induced VC. Scale bar, 100
HPV E6 oncoprotein targets histone methyltransferases for modulating specific. Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu,
 1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
Inventory of Supplemental Information for Melkman-Zehavi et al.
 Inventory of Supplemental Information for Melkman-Zehavi et al. Supplementary methods for Melkman et al. Supplementary Figures Figure S1, related to Figure 2 Islet size and total beta cell mass of mutant
Inventory of Supplemental Information for Melkman-Zehavi et al. Supplementary methods for Melkman et al. Supplementary Figures Figure S1, related to Figure 2 Islet size and total beta cell mass of mutant
Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of
 Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Supplementary Figure 1. Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings.
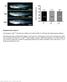 Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Supplementary information
 Supplementary information Supplementary figures Figure S1 Level of mycdet1 protein in DET1 OE-1, OE-2 and OE-3 transgenic lines. Total protein extract from wild type Col0, det1-1 mutant and DET1 OE lines
Supplementary information Supplementary figures Figure S1 Level of mycdet1 protein in DET1 OE-1, OE-2 and OE-3 transgenic lines. Total protein extract from wild type Col0, det1-1 mutant and DET1 OE lines
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Protocol. sirna transfection methodology and performance
 Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
Supplementary Figure 1. α-synuclein is truncated in PD and LBD brains. Nature Structural & Molecular Biology: doi: /nsmb.
 Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss
 SUPPLEMENTARY INFORMATION Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss Yunjong Lee, Senthilkumar S. Karuppagounder, Joo-Ho Shin, Yun-Il Lee, Han Seok Ko, Debbie Swing,
SUPPLEMENTARY INFORMATION Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss Yunjong Lee, Senthilkumar S. Karuppagounder, Joo-Ho Shin, Yun-Il Lee, Han Seok Ko, Debbie Swing,
Confocal immunofluorescence microscopy
 Confocal immunofluorescence microscopy HL-6 and cells were cultured and cytospun onto glass slides. The cells were double immunofluorescence stained for Mt NPM1 and fibrillarin (nucleolar marker). Briefly,
Confocal immunofluorescence microscopy HL-6 and cells were cultured and cytospun onto glass slides. The cells were double immunofluorescence stained for Mt NPM1 and fibrillarin (nucleolar marker). Briefly,
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development
 Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
ASPP1 Fw GGTTGGGAATCCACGTGTTG ASPP1 Rv GCCATATCTTGGAGCTCTGAGAG
 Supplemental Materials and Methods Plasmids: the following plasmids were used in the supplementary data: pwzl-myc- Lats2 (Aylon et al, 2006), pretrosuper-vector and pretrosuper-shp53 (generous gift of
Supplemental Materials and Methods Plasmids: the following plasmids were used in the supplementary data: pwzl-myc- Lats2 (Aylon et al, 2006), pretrosuper-vector and pretrosuper-shp53 (generous gift of
7.06 Cell Biology EXAM #2 March 20, 2003
 7.06 Cell Biology EXAM #2 March 20, 2003 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #2 March 20, 2003 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
indicated numbers of pups at day of life (DOL) 10, or embryonic day (ED) B. Male mice of
 SUPPLEMENTRY FIGURE LEGENDS Figure S1. USP44 loss leads to chromosome missegregation.. Genotypes obtained from the indicated numbers of pups at day of life (DOL) 10, or embryonic day (ED) 13.5.. Male mice
SUPPLEMENTRY FIGURE LEGENDS Figure S1. USP44 loss leads to chromosome missegregation.. Genotypes obtained from the indicated numbers of pups at day of life (DOL) 10, or embryonic day (ED) 13.5.. Male mice
TRANSGENIC ANIMALS. -transient transfection of cells -stable transfection of cells. - Two methods to produce transgenic animals:
 TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
Replication-independent chromatin loading of Dnmt1 during G2 and M phases
 Replication-independent chromatin loading of Dnmt1 during G2 and M phases Hariharan P. Easwaran 1, Lothar Schermelleh 2, Heinrich Leonhardt 1,2,* and M. Cristina Cardoso 1,* 1 Max Delbrück Center for Molecular
Replication-independent chromatin loading of Dnmt1 during G2 and M phases Hariharan P. Easwaran 1, Lothar Schermelleh 2, Heinrich Leonhardt 1,2,* and M. Cristina Cardoso 1,* 1 Max Delbrück Center for Molecular
Supplemental Figure S1. PGRN Binding to Sortilin.
 1 Neuron, volume 68 Supplemental Data Sortilin-Mediated Endocytosis Determines Levels of the Frontotemporal Dementia Protein, Progranulin Fenghua Hu, Thihan Padukkavidana, Christian B. Vægter, Owen A.
1 Neuron, volume 68 Supplemental Data Sortilin-Mediated Endocytosis Determines Levels of the Frontotemporal Dementia Protein, Progranulin Fenghua Hu, Thihan Padukkavidana, Christian B. Vægter, Owen A.
a) Stock 4: 126 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males.
![a) Stock 4: 126 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males. a) Stock 4: 126 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males.](/thumbs/88/116538069.jpg) Drosophila Problem Set 2012 1) You have created P element transformants of a construct that contains the mini-white gene, which confers an orange eye color in a homozygous white mutant background. For
Drosophila Problem Set 2012 1) You have created P element transformants of a construct that contains the mini-white gene, which confers an orange eye color in a homozygous white mutant background. For
Supplementary Methods
 Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
Giardia RNAi Paper Discussion. Supplemental - VSG clonality. Variant-specific surface protein VSP9B10
 Giardia RNAi Paper Discussion Supplemental - VSG clonality VSP9B10 Green - VSP9B10 antibody Blue - DAPI Demonstration that cell line expresses a single VSP Variant-specific surface protein Outside Inside
Giardia RNAi Paper Discussion Supplemental - VSG clonality VSP9B10 Green - VSP9B10 antibody Blue - DAPI Demonstration that cell line expresses a single VSP Variant-specific surface protein Outside Inside
