Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/
|
|
|
- Barrie Greene
- 6 years ago
- Views:
Transcription
1 Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/ catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek Luca Caneparo, 1 Ya-Lin Huang, 3,4 Nicole Staudt, 1,4 Masasumi Tada, 2,4 Reiner Ahrendt, 1 Olga Kazanskaya, 3 Christof Niehrs, 3 and Corinne Houart 1,5 1 Medical Research Council Centre for Developmental Neurobiology, King s College London, SE1 1UL London, United Kingdom; 2 Anatomy and Developmental Biology Department, University College London, WC1E 6BT London, United Kingdom; 3 Division of Molecular Embryology, German Cancer Research Center, D Heidelberg, Germany Dickkopf-1 (Dkk1) is a secreted protein that negatively modulates the Wnt/ catenin pathway. Lack of Dkk1 function affects head formation in frog and mice, supporting the idea that Dkk1 acts as a head inducer during gastrulation. We show here that lack of Dkk1 function accelerates internalization and rostral progression of the mesendoderm and that gain of function slows down both internalization and convergence extension, indicating a novel role for Dkk1 in modulating these movements. The motility phenotype found in the morphants is not observed in embryos in which the Wnt/ catenin pathway is overactivated, and that dominant-negative Wnt proteins are not able to rescue the gastrulation movement defect induced by absence of Dkk1. These data strongly suggest that Dkk1 is acting in a catenin independent fashion when modulating gastrulation movements. We demonstrate that the glypican 4/6 homolog Knypek (Kny) binds to Dkk1 and that they are able to functionally interact in vivo. Moreover, Dkk1 regulation of gastrulation movements is kny dependent. Kny is a component of the Wnt/planar cell polarity (PCP) pathway. We found that indeed Dkk1 is able to activate this pathway in both Xenopus and zebrafish. Furthermore, concomitant alteration of the catenin and PCP activities is able to mimic the morphant accelerated cell motility phenotype. Our data therefore indicate that Dkk1 regulates gastrulation movement through interaction with LRP5/6 and Kny and coordinated modulations of Wnt/ catenin and Wnt/PCP pathways. [Keywords: Dickkopf-1; HSPG; Wnt/PCP; gastrulation movements] Supplemental material is available at Received August 15, 2006; revised version accepted December 22, These authors contributed equally to this work. 5 Corresponding author. corinne.houart@kcl.ac.uk; FAX Article is online at The anterior brain is first defined as a specific portion of rostral neural ectoderm expressing a set of early neural markers such as hesx1/anf1 and otx2. This initial territory is progressively refined into specific presumptive midbrain and forebrain areas (Wilson and Houart 2004). The anterior posterior (AP) patterning of the CNS is thought to arise, in early gastrula, through the action of posteriorizing signals, coming from the newly formed mesoderm, on an anterior neural ectoderm (Stern 2001). The anterior neural plate therefore needs to be protected from posteriorizing influence to maintain rostral neural identities such as forebrain and midbrain. This is achieved by both moving away from, and expressing antagonists to, these signals (Wilson and Houart 2004). Among the most studied posteriorizing signals are the secreted Wnt molecules (Erter et al. 2001; Kiecker and Niehrs 2001a; Lekven et al. 2001). Wnts are secreted by the mesendoderm at the marginal zone, as gastrulation proceeds and gastrula embryos with increased Wnt activity fail to develop rostral neural identity and show a posterior transformation of the anterior neural plate (Kim et al. 2000; Kiecker and Niehrs 2001b; Lekven et al. 2001). A variety of molecules, acting as secreted antagonists of the Wnt pathway, have been identified in the Spemann Organizer. Most of them are related to the extracellular domain of the Wnt receptor Frizzled and act by direct binding to Wnt proteins (Leyns et al. 1997; Wang et al. 1997; Hsieh et al. 1999; Kawano and Kypta 2003). GENES & DEVELOPMENT 21: by Cold Spring Harbor Laboratory Press ISSN /07; 465
2 Caneparo et al. Distinct from these, the Dickkopf family of secreted molecules (Dkk) (Glinka et al. 1998; Kawano and Kypta 2003) influences the reception of Wnt signals by binding to the Frizzled coreceptors LRP5/6 trans-membrane proteins (Mao et al. 2001) and Kremen (Mao et al. 2002). Among them, Dkk1 is shown to have a strict inhibitory effect on Frizzled receptors (Kazanskaya et al. 2000). It is first expressed in the forming mesendoderm of the late zebrafish, Xenopus, and mouse blastula and then localized specifically in the nascent Spemann Organizer of the early gastrula (Kazanskaya et al. 2000; Shinya et al. 2000). Moreover, both in zebrafish and mouse, dkk1 is expressed in early extra-embryonic tissue (yolk syncytial layer [YSL] and anterior visceral endoderm [AVE]). In frog and fish, Dkk1 overexpression is able to anteriorize neural tissue (Kazanskaya et al. 2000; Shinya et al. 2000). It can also induce a secondary head if coexpressed with BMP antagonists in ventral blastomeres of Xenopus early blastula embryos. These data led to the conclusion that Dkk1 acts as a head inducer through inhibition of the Wnt/ catenin posteriorizing activity in early gastrula embryos (Niehrs et al. 2001). Requirement for Dkk1 function in head formation has been further supported by the characterization of mouse embryos lacking Dkk1 gene function. Indeed, the genetic knockout of the mouse dkk1 leads to the formation of headless embryos due to lack of maintenance of anterior neural identity during gastrulation (Mukhopadhyay et al. 2001). Interestingly, failure in establishing the forebrain territories in these embryos is not due to requirement of Dkk1 in the AVE, as mosaic embryos with a dkk1 / AVE but containing a functional dkk1 gene in all other tissues develop normally (Mukhopadhyay et al. 2001). Thus, Dkk1 has been undoubtedly shown to be required for establishment of the forebrain in vertebrates, and its biochemical properties have been reasonably well understood (van Tilbeurgh et al. 1999). However, the mechanism by which Dkk1 acts on the neural ectoderm to support forebrain formation is yet to be unraveled. Late in gastrulation, local inhibition of Wnt signaling is also required to maintain telencephalon and eye identities inside the vertebrate anterior neural plate (Heisenberg et al. 2001; Houart et al. 2002; Kim et al. 2002; Lagutin et al. 2003). One of Dkk1 s possible functions may therefore be to ensure the expression of specific secreted Frizzled-Related Proteins (sfrps) such as tlc inside the anterior neural border (ANB). We found that such is the case, as lack of Dkk1 dramatically reduces the expression of tlc (L. Caneparo, R. Ahrendt, J. Peres, M. Kapsimali, and C. Houart, in prep.). In this study, we address the role of Dkk1 in the mesendoderm during gastrulation. Dkk1 is thought to act through vertical signaling from the anterior mesendoderm (Kiecker and Niehrs 2001b; Niehrs et al. 2001). We show that in zebrafish embryos with little or no Dkk1 activity, very little change in the molecular identity of the internalized axial tissue is observed, suggesting that Dkk1 protein may also act on planar signaling events prior to internalization. More importantly, our data show that gain and loss of Dkk1 function both lead to defects in gastrulation movements. Indeed, lack of Dkk1 function accelerates internalization and rostral progression of the mesendoderm, while gain of function slows down both internalization and convergence extension. These findings point to a role for Dkk1 in modulating these movements. Surprisingly, we show that upregulation of the catenin pathway is not inducing the gastrulation movement defects observed in the Dkk1 morphants, strongly suggesting that Dkk1 is able to interact with another signaling pathway. We therefore tested the possible interaction of Dkk1 with known regulators of gastrulation movements. We demonstrate that Dkk1 is able to bind in vivo to the glypican4/6 member Knypek (Kny, a Dally-like homolog). We find that Kny and Dkk1 are able to potentiate each other s activity when coexpressed in early embryos. Complementarily, propagation of the secreted Dkk1 is greatly reduced in kny mutants. These data demonstrate that propagation and range of action of Dkk1 are likely to be dependent on interaction with the heparan sulfate proteoglycan (HSPG) Kny. Potentiation of Kny function by Dkk1 and Kny requirement for acceleration of gastrulation movements in the Dkk1 morphants together strongly suggest that Dkk1 cooperates with Kny in regulating gastrulation movements. We therefore tested whether Dkk1 was able to modulate the Wnt/planar cell polarity (PCP) pathway. Our results show that Dkk1 overexpression mimics up-regulation of the Wnt/PCP pathway both in Xenopus and zebrafish and dramatically increases JNK phosphorylation, strongly suggesting that Dkk1 is positively modulating the Wnt/PCP pathway. However, decrease of Wnt/PCP activity in the Dkk1 morphants is not the only cause of the gastrulation movement phenotype observed, as loss of PCP function in vertebrates is not associated with acceleration of movements. In fact, we find that increase of Wnt/ catenin activity accompanied by mild down-regulation of the Wnt/PCP pathway is a condition for which acceleration of internalization is often observed. We therefore propose that Dkk1 modulates gastrulation movements by coordinated modulation of the Wnt// catenin and PCP pathways, through interaction with both Kny and the LRP/Kremen complex. All together, our results led us to propose a model by which Dkk1, via endocytosis of LRP5/6, may transform the biochemical properties of the Frizzled receptors and/or an interacting cytoplasmic component from Wnt/ catenin to a Wnt/PCP conformation, thereby up-regulating the latter pathway while inhibiting the canonical one. Results Dkk1 modulates gastrulation movements To study the possible role of Dkk1 in induction of the anterior neural border signaling center (Houart et al. 1998), we used specific antisense morpholino oligonucleotides (Nasevicius and Ekker 2000) that nearly completely inhibit Dkk1 translation in injected zebrafish embryos (Fig. 1G J). The morphant embryos show a severe reduction of forebrain territories (Fig. 1A 466 GENES & DEVELOPMENT
3 Dkk1 binds Kny and regulates gastrulation movements F), as predicted by previous studies of Dkk1 loss of function in Xenopus and mouse (Glinka et al. 1998; Mukhopadhyay et al. 2001). It has been strongly suggested that vertical signals emanating from the anterior axial mesendoderm/prechordal plate are required for the establishment of the anterior neural territory (Kazanskaya et al. 2000; Kiecker and Niehrs 2001b) and that Dkk1 is one of the key players in this signaling function. However, in the absence of dkk1 in both fish (L. Caneparo, R. Ahrendt, J. Peres, M. Kapsimali, and C. Houart, in prep.) and mouse (Mukhopadhyay et al. 2001), otx2 is properly induced in the anterior neural territory, suggesting that some initial patterning events occur in the absence of Dkk1. In Dkk1 antibody injected Xenopus embryos hhex and Blimp1, two anterior endoderm markers and gsc and shh in prechordal plate are down-regulated (Kazanskaya et al. 2000). In the mouse dkk1/noggin double mutants, Hesx1 expression is down-regulated in the presumptive prechordal plate (del Barco et al. 2003). These results indicate that the most rostral population of axial mesendoderm is impaired in the absence of dkk1. In order to assess whether the zebrafish Dkk1 morphant (dkk1mo) embryos also present some axial mesendodermal defects, we analyzed the expression pattern of a set of axial markers (Fig. 2A H; data not shown). No change in expression has been found (between 41 and 70 injected embryos tested for seven axial markers at 60% 65%, 80% 90% epiboly and bud stages) except for a significant reduction of the anterior prechordal plate marker gsc (n = 17/58) (Fig. 2G,H), confirming the defect observed in Xenopus and mouse. In the course of this analysis, we noticed a striking difference in position of the rostral-most limit of the axial mesendoderm (Fig. 2A F). This observation has been made looking at the expression of a set of axial markers and suggested acceleration in axial mesendoderm progression. In order to address this possibility, we followed the movement of the axial mesendoderm in live transgenic embryos containing the insertion of a gene coding for the green fluorescent protein (GFP) under the control of the gsc promoter (n = 48/55) (Fig. 2Q Y). In wild-type embryos, the rostral tip of the internalized mesendoderm is progressively moving toward the animal pole during gastrulation and reaches the pole by 90% epiboly. In dkk1mo embryos, the rostral axial mesendoderm reaches the animal pole by 75% epiboly (Fig. 2U,V), min earlier than in wild-type embryos raised at 28 C. We concurrently observed that dkk1mo embryos show a slightly narrower anterior neural plate by the end of gastrulation, although no defect in BMP signaling has been detected rostrally (n = 31/31, Fig. 2N,O), and no anterior neural plate size difference is visible earlier in gastrulation (n = 35/35) (Fig. 2K,L). Finally, the rostral limit of expression of snail1a (data not shown) and sprouty4 (Fig. 2I,J) in nonaxial mesendoderm is also shifted anteriorly in morphant embryos, due to change in cell identity and/or cell motility. Both patterning and motility phenotype observed in the morphants can be corrected by coinjection of both dkk1mo and dkk1 full-length transcripts lacking the MO target sequence (Supplementary Fig. S1). These observations suggest that although epiboly movements are not visibly perturbed in dkk1mo morphants, some gastrulation movements are accelerated in these embryos. In order to test this further, we assessed directionality and speed of mesendodermal movements in Dkk1 morphant embryos. We first assessed movements of lateral Figure 1. dkk1mo efficiently represses dkk1 translation, which induces severe reduction of the forebrain. (A F) Loss of Dkk1 function represses formation of eye and telencephalon. Lateral views, anterior to the left, of heads of wild-type (A,C,E) and dkk1moinjected (B,D,F) embryos. (G J ) Rescue of dkk1gfp overexpression by Dkk1MO. (G J) Lateral view of live 80% epiboly (under UV illumination, G J ) and prim5 (G,J) embryos after one-cell-stage injection of 50 ng/µl dkk1gfp alone (G,G ) or together with 0.4 mg/ml (H,H ), 1 mg/ml (I,I ), and 1.5 mg/ml (J,J ) Dkk1MO. With the exception of C and D, the embryos shown in this figure are alive. Bar, 100 µm. GENES & DEVELOPMENT 467
4 Caneparo et al. Figure 2. Dkk1 regulates gastrulation movements. Lateral (A,B,E,F,I Y), dorsal (C,D), and animal pole (G,H) views of control (A,C,E,G,I,K,N,Q S), Dkk1MO-injected (B,D,F,H,J,L,O,T V), and dkk1rna-injected (M,P,W Y) gastrula embryos. A J and Q Y show progression of the mesendoderm by markers in fixed embryos (A J) and by observation of the GFP-expressing axial mesendoderm in live embryos (Q Y). The black arrowhead indicates the position of the rostral-most mesendoderm. The red frame highlights the stages at which the difference in mesendoderm progression is obvious in the live Dkk1 morphants. (K P) Lateral views of, in blue, neural plate (K M) and epidermal (N P) territories in wild-type (K,N), dkk1mo (L,O), and dkk1rna (M,P) embryos. Red arrowhead shows the caudally shifted anterior edge of the neural territory. mesoderm by transplanting in the germ ring, on either side of the shield (at 35 from it), rhodamin-labeled wildtype and fluorescein-tagged dkk1mo cells in a dkk1mo shield stage host embryo. We followed their progression by confocal time-lapse analysis (see Materials and Methods; n = 56, Fig. 3A C). In most cases, dkk1mo cells move faster toward the animal pole than the wild-type clones (n = 48/56). A difference in cell behavior is visible from the onset of gastrulation as dkk1mo cells move under the epiblast more frequently than wild-type cells (Fig. 3B, graph in I). This acceleration does not seem to be dependent on the clone size (seen for wild-type and dkk1mo clones of either five, 15, or 25 transplanted cells). Expectedly, wild-type and dkk1mo clones move in very similar ways if transplanted in wild-type hosts (n = 25/25) (Fig. 3D), as secreted Dkk1 from host cells almost certainly compensates for the absence of protein in the dkk1mo grafted cells. Similarly, if wild-type and dkk1mo clones are grafted adjacent inside the shield of dkk1mo host embryos (Fig. 3E,F), most cells are moving together (defective cells receiving Dkk1 from secreted wild-type neighbors), with some dkk1mo cells running in front and wild-type cells trailing behind (n = 9/16 and 7/16 without a significant difference in position between the two cell types). To quantify further the changes observed in the mor- 468 GENES & DEVELOPMENT
5 Dkk1 binds Kny and regulates gastrulation movements Figure 3. More frequent mesendoderm internalization and faster rostral progression in the Dkk1 morphants and slowed-down internalization and CE defects in Dkk1 gain of function. (A H) Dorsal (B F), animal pole (A), and lateral (G,H) views of dkk1mo (A C,E,F) or wild-type (D,G,H) embryos in which wild-type (red in live embryos and brown in fixed specimen) and dkk1mo (green in live embryos and dark blue in fixed specimen) cells have been transplanted inside the germ ring at onset of gastrulation. A C show the same embryo at progressively later stages of gastrulation. Note that H shows transplanted dkk1rna-expressing cells in dark blue. Dashed line indicates the midline. (I) Timing of internalization of 15 wild-type (blue) and dkk1mo (red) cells transplanted in the margin of early gastrula hosts. Measure of transplants in the lateral margin are represented by full dots and transplants in the axial margin (shield) are represented by empty dots. X represents time and Y represents the number of cells. (J) Measure of the AP length of the transplanted cell population at bud stage in microns (Y axis). Wild-type clones are shown in blue, dkk1mo are shown in red, and dkk1rna are shown in green. (K) Measure of the distance of the leading transplanted cell from the embryo margin at 95% epiboly in microns (Y axis). Wild-type clones are shown in blue, dkk1mo are shown in red, and dkk1rna are shown in green. (L) Expression of dkk1 in the marginal mesendoderm. Animal pole view of a shield stage wild-type embryo. (S) Shield (Spemann Organizer). Note the absence of transcript in the ventral margin and the graded level of expression from dorsal (S, shield) to lateral. (M,N) Cell shape and cohesion in the lateral (M) and dorsal (N) mesendoderm (arrows) as it internalizes in a live shield stage embryo. Inset in M shows a close-up of the internalizing lateral mesendoderm cell, emphasizing the mesenchymal shape of the lateral mesendoderm cells. phant gastrulae, we followed the progression of 15 cells transplanted into the germ ring of a morphant host and took three sets of measurements: the number of internalized cells/minute (Fig. 3I), the AP length of the clone at 90% epiboly (Fig. 3J), and the distance of the rostralmost cell from the posterior margin at 95% 100% GENES & DEVELOPMENT 469
6 Caneparo et al. epiboly (Fig. 3K). In our experimental conditions, wildtype transplanted cells internalize at an average of 0.55 cell per minute in lateral and axial mesoderm (Fig. 3I, blue dots/circles), while morphant cells move inward at an average of 0.85 cell per minute in lateral mesoderm and 1.8 cell per minute in the axial mesendoderm (Fig. 3I, red dots/circles). The AP length of morphant clones at 90% epiboly is slightly bigger than wild type, strongly suggesting that convergence/extension (CE) movements are not impaired in the absence of Dkk1 function (Fig. 3J). However, the position of the leading cell inside the clones is significantly shifted anteriorly in morphants compared with wild type (Fig. 3K), indicating that rostral progression is increased when Dkk1 is lacking. Indirectly, this finding (similar clone shape and length but shifted rostrally) suggests that more cells are internalized in the morphants. Lack of Dkk1 function therefore favors mesendodermal internalization and rostral progression, indicating that Dkk1 protein normally negatively regulates this type of movement. We next assessed the effect the overexpression of dkk1 has on cell movement, using the same approaches. A lag in progression of the axial mesendoderm is observed (Fig. 2W Y), and a caudal displacement of the anterior limit of the neural plate (Fig. 2M,P, arrowheads) typical of embryos defective in extension movements. Clonal analysis of cell motility shows that dkk1 overexpressing clones present an increase in cell dispersion and a slowing down of internalization, rostral progression, and convergence (n = 39/39) (Fig. 3,H; green graph in J,K). All together, these data indicate that Dkk1 modulates gastrulation movements. Gain of Wnt/ catenin function does not lead to acceleration of mesendoderm internalization As Dkk1 regulates negatively the Wnt/ catenin pathway, we tested whether the acceleration of gastrulation movements in the morphant embryos is the consequence of up-regulation of this pathway. We first addressed whether mesendodermal progression was affected in the mbl mutant embryos, lacking axin1 function (Heisenberg et al. 2001) and therefore unable to degrade catenin. We find no visible difference, either by measuring the extent of the lefty1 expression in axial mesendoderm or when monitoring cell movements of labeled mesendoderm (data not shown). To ascertain that the absence of modulation of movement was not caused by a relatively mild increase in catenin activity in mbl, we tested whether any change was detectable in embryos lacking both mbl and tcf3 function (injection of tcf3 and tcf3b morpholino [Dorsky et al. 2003] in mbl) (Fig. 4). In these embryos, we have been unable to observe any increase in cell movement. Conversely, we observed a slight reduction of axial mesendoderm progression in 29% of the double mbl / ; tcf3mo embryos (n = 8/28) (Fig. 4D). Finally, NWnt8 overexpression, obtained by RNA injection at the one-cell stage (25 pg/ embryo, n = 37) (Fig. 4J) is unable to rescue the acceleration observed in the Dkk1 morphants. All together, these data strongly suggest that the influence of Dkk1 on gastrulation movements is at least in part Wnt/ catenin independent. However, this pathway seems to regulate some aspects of cell cohesion, as we observed that cells from clones lacking both mbl and tcf3 function were losing touch with each other faster than wild type after transplantation (clone shape at 75% 80% epiboly) (Fig. 4G,H). To quantify further the changes in cell movements imposed by gain of Wnt/ catenin activity, we used our transplantation approach again and transplanted wnt8 mrna-injected donor cells into the margin (35 from the shield) of early shield stage wild-type host embryos and compared with wild-type transplants in the same conditions (either transplanted on the other side of the same hosts or in different host embryos). A series of wnt8 doses has been tested (Fig. 4K N). The wnt8-expressing cells internalized as well as the wild-type cells, except for the cells expressing the highest dose, for which delay in progression and CE defect begins to be observed (Fig. 4N, graph in O,P), reminiscent of the defects observed in Xenopus (Kühl et al. 2001). In most cases, the clones are more dispersed (Fig. 4L N). Finally, a very small number of wnt8-expressing cells (from set of 3 pg of transcripts/donor) are very rarely detected slightly further away from the margin than in wild type (n =1/ 74) (data not shown). These results indicate that Wnt/ catenin activity is not readily able to induce acceleration of mesendoderm internalization and progression, but confirm the less cohesive nature of cells exposed to high level of Wnt/ catenin signaling. This observation nicely correlates with the distinct cell adhesion properties found in the lateral (noncohesive) (Fig. 3M) and dorsal (cohesive) (Fig. 3N) mesendoderm, which are exposed to low and high levels of Dkk1, respectively (Fig. 3L). Dkk1 is therefore likely to regulate cohesion through modulation of the Wnt/ catenin pathway. The glypican4/6 Knypek is able to bind to Dkk1 and potentiates its activity in vivo In vertebrates, a subset of gastrulation movements are regulated by the Wnt/PCP pathway (for reviews, see Tada et al. 2002; Wallingford 2005). Both Wnt5 and Wnt11 are signaling molecules required for proper convergence extension (Heisenberg et al. 2000; Kilian et al. 2003) through activation of pathways including the JNK and Ca ++. One possible way Dkk1 may regulate cell movements is through modulation of the PCP pathway. Such a modulation may be indirect, through repression of the Wnt/ catenin (Kühl et al. 2001). Alternatively, it may be direct, by interaction with PCP pathway components. Since Dkk1 binds heparan (Fedi et al. 1999), we tested if a related molecule, Knypek, a member of the HSPG family, required for Wnt/PCP activity (Topczewski et al. 2001), is able to interact with Dkk1. We tested whether Dkk1 and Knypek are able to bind each other, using immunoprecipitation techniques. We tested membrane colocalization and binding between these two molecules in a cell culture system, by transfection of an expression vector containing a Flag-tagged 470 GENES & DEVELOPMENT
7 Dkk1 binds Kny and regulates gastrulation movements Figure 4. Gain of Wnt/ catenin activity does not phenocopy the gastrulation movement defect seen in the Dkk1 morphants. (A F) Lateral views of live prim5 (A,C,E) or dorsal views of lefty1 exression in fixed 90% 95% epiboly (B,D,F), wild-type (A,B), and mbl mutant injected with tcf3 + 3bMO (C,D), and dkk1mo (E,F) embryos. (G,H) Clone shape of transplanted wild type (G) ormbl mutant injected with tcf3 + 3bMO (H) in the host embryo at 75% 80% epiboly. (I,J) Lateral views of 75% 80% epiboly gscgfp transgenic live embryo (I) ordkk1mo coinjected with 20 pg of dominant-negative wnt8 RNA (J), showing the GFP-expressing axial mesendoderm in green under UV illumination. (K N) Lateral views of 90% epiboly embryos showing the distribution of the wnt8-expressing cells (blue) transplanted at shield stage. The dose injected per embryo is indicated in the bottom right corner. (O) Timing of internalization of 25 wild-type (blue) and wnt8-expressing (10 pg, pink; 25 pg red; 40 pg purple) cells transplanted in the margin of early gastrula hosts. X represents time and Y represents the number of cells. (P) Measure of the distance of the leading transplanted cell from the embryo margin at 100% epiboly in microns (Y axis). Wild-type clones are shown in blue and wnt8-expressing clones are shown in pink (10 pg), red (25 pg), and purple (40 pg). knypek c-dna sequence (kny-flg) (Topczewski et al. 2001) and/or another containing a dkk1gfp fusion molecule, coding for an active fluorescent Dkk1 protein (see Fig. 1G,G ). We assessed localization of the two proteins in these transfected cells. Protein extracts from transfected cells were precipitated with either an anti-flag or an anti-gfp antibody. The precipitates were run on acrylamide gel and Western blot immunostained either with GFP or Flag antibody. The blots show that in extracts from cells cotransfected with both dkk1gfp and kny-flg DNA (or from a mixed culture of dkk1gfp-expressing and kny-flg-expressing cells), Kny-Flg proteins are detected in the anti-gfp precipitates (Fig. 5A), unequivocally showing that Dkk1 is able to bind to Knypek in cell culture conditions. By the same technique, we also show this binding in gastrulae extracts (Fig. 7J, below), opening the possibility that Dkk1 may directly modulate Knypek-dependent signaling events in vivo. GENES & DEVELOPMENT 471
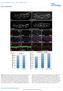
8 Caneparo et al. Figure 5. Knypek binds to Dkk1, is required for its propagation, and potentiates its effects. (A) Western blot of an immunoprecipitation assay. Protein extracts from cells transfected with different combinations of DNA (+) are either run untreated (last three lanes) or first immunoprecipitated with an anti-gfp antibody (first five lanes), then run on a gel. The gel is then transferred on filter and stained with an anti-flag antibody (detecting the Flag-tagged Knypek protein) or an anti- GFP antibody (detecting the GFP [1] or dkkgfp fusion [2] proteins). (Lane 4) Red arrowshows the presence, after GFP immunoprecipitation, of Kny-Flag proteins in extracts from cells expressing both Dkk1GFP and KnyFlag. (B E ) Lateral views, rostral to the left, of prim5 live wild-type (B), kny RNA-injected (C), dkk1rna-injected (D), and kny + dkk1rna-injected (E,E ) embryos. In E, the quantity of RNA injected is identical to the that used for the single injections in C and D. The phenotype shown in E and E are found in 31% and 64% of the injected embryos, respectively. (F I) Animal pole views (insets) and lateral views of live or fixed (insets) wild type (F,G) and kny homozygous (H,I) injected with control (F,H) ordkk1 morpholino (G,I). Insets show expression patterns of hgg1 (rostral mesendoderm, hg) and brachyury (in the notochord, n). Arrowhead shows rostral end of the body axis. (J M) Visualization of the dkk1gfp molecules (in green) secreted by the transplanted dkk1gfp-expressing wild-type cells (yellow) in wild-type (J,K) and kny / (L,M) hosts. K and M are highmagnification views of the distribution of secreted proteins away from the graft (or its absence at a distance from the clone in M). Note that the cell nuclei are visible as dark discs in unlabeled cells. The wildtype dkk1gfp-expressing cells are very intensely fluorescent when placed in the kny / mutant, masking the nucleus in these cells. (N P) Lateral views, rostral to the left, of wild-type (N) orkny / (O,P) embryos, untreated (inset in N,O) or in which cells from a dkk1 RNA-injected donor embryo (in brown) have been transplanted in the shield at 50% epiboly (N,P). Note the shorter tail and big eye and brain in N. (P) No change in eye size is ever observed in transplanted kny / embryos; the brain size is generally comparable to untransplanted mutants. (Q Z) Lateral views of live embryos (Q V) or dorsal views of fixed gastrulae (W Z). All embryos come from a kny +/ cross injected with either 1.8 pg of kny transcripts (Q S,W,X) or 1.8 pg of kny and2pgofdkk1 transcripts (T V,Y,Z). The number of embryos showing each illustrated phenotype is given in the bottom right corner of each picture. In W Z, hgg1 expression shows rostral mesendoderm (hg) and brachyury, the notochord (n). The defects in cell movements observed in Dkk1 gain or loss of function may therefore be due to modulation of a Knypek-dependent signaling activity. We tested this hypothesis by assessing whether embryos carrying a null mutation in the kny gene are losing the ability to respond to the absence of Dkk1 function. If Dkk1 acts through a kny independent way, Dkk1 lack of function may rescue part of the movement defects observed in the kny / embryos. We observed that the embryos lacking both Dkk1 and Knypek show delay in movements identical to the ones observed in single kny / embryos (n = 31/31 fixed kny / embryos and n = 15/15 kny / live embryos) (Fig. 5F I). This suggests that either Dkk1 influence on motility may depend on its direct binding to Knypek or that Knypek is functionally downstream from Dkk1 in the same developmental process. To attempt to discriminate between these two possibilities, we tested whether Kny could increase Dkk1 activity when misexpressed in wild-type embryos. We injected kny or dkk1 transcripts alone or together in wildtype embryos, choosing a quantity of transcripts that gives no (for kny) (Fig. 5C) or weak (for dkk1) (Fig. 5D) 472 GENES & DEVELOPMENT
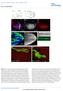
9 Dkk1 binds Kny and regulates gastrulation movements phenotype when injected alone. Embryos injected with the same or half the dose of both transcripts (Supplementary Table 1), develop an enormous head and the short body axis typical of a severe dkk1 overexpression (Fig. 5E,E ; Supplementary Table 1). These results indicate that Dkk1 and Kny can act together in the same developmental process. Finally, we analyzed the localization of Dkk1 protein in wild-type and kny / embryos, using the dkk1gfp fusion molecule described above. We transplanted cells from dkk1gfp-injected wildtype donor embryos into shield stage wild-type embryos or progenies of kny /+ fish. We then followed the expression of the fusion proteins under the confocal microscope. kny / hosts are identified based on their phenotype at 24 h post-fertilization (hpf). We first observed that propagation of the Dkk1GFP proteins is extremely fast (an average of 21 cell diameters after 45 min) in wild-type embryos (data not shown). Moreover, very intense small and highly dynamic fluorescent dots are visible inside the host cells, strongly suggesting an endocytic pathway (Fig. 5K; also suggested by Mao et al. 2002). More importantly, we found extensive propagation of the secreted molecule in 23/24 of the wild-type embryos while no propagation has been detected in 8/9 kny / analyzed (Fig. 5J M). In the absence of propagation, the fluorescence is detected weakly around the cells and highly inside the cytoplasm. Kny is therefore required for propagation of Dkk1. To assess whether this propagation is necessary for Dkk1 function, we asked if propagation of the Dkk1 molecules was required for its well-described anteriorizing function. We therefore transplanted donor dkk1-expressing cells (from dkk1 mrna-injected donors embryos) into the shield (Spemann Organizer) of early gastrula wild-type or kny hosts. We observed that grafted dkk1-expressing cells are able to induce a big head/ short trunk phenotype in wild type (n = 19/21) (Fig. 5N). Introduction of Dkk1-overexpressing cells in the wildtype shield is therefore able to affect the general AP patterning such that transplanted embryos always show reduction of the trunk and the giant eyes typical of the Dkk1-injected individuals. We were unable to observe an increase of the eye and overall brain in kny embryos (n = 0/23) (Fig. 5P), but we sometimes observed a slight increase in the size of the telencephalon (n = 6/23), probably due to the effect of Dkk1 from donor cells when in close vicinity of the forebrain. Finally, we directly assess whether Dkk1 is able to cooperate with Kny in regulating gastrulation movements. To this aim, we tested whether Dkk1 is able to help Kny in rescuing the kny mutant phenotype. We injected, at the one-cell stage, the progeny of kny +/ parents with a suboptimal quantity of kny full-length RNA alone or accompanied by a low dose (alone not inducing a phenotype) of dkk1 transcript. To our surprise, the coinjected embryos showed a robust rescue (Fig. 5Q Z), strongly suggesting that Kny and Dkk1 act similarly on the same gastrulation movements. As Dkk1 alone is unable to rescue kny mutant embryos (n = 32) (data not shown), the double rescue indicates that Dkk1 and Kny control cell motility together through regulation of the same pathway(s). All together, the results in this section show that (1) Dkk1 is able to bind to Kny and requires Kny for its propagation, (2) propagation is necessary for Dkk1 function, and (3) Dkk1 and Kny can act together to regulate gastrulation movements. Dkk1 promotes the activity of the Wnt/PCP pathway As Dkk1 is able to bind to Knypek and enhance its function, it may directly regulate the Wnt/PCP pathway. We first tested whether Dkk1 negatively modulates Wnt/ PCP activity. To address this possibility, we examined if the acceleration of rostral progression observed in the Dkk1 morphants may rescue the gastrulation phenotype observed in slb embryos, homozygous for a null mutation in the wnt11 gene (Heisenberg et al. 2000). CE defects were not rescued in slb / embryos (obtained from slb / homozygous parents) injected with dkk1mo (n = 41) (Fig. 6A C ; data not shown). However, we observed some change in the AP extent of the prechordal territory marked by gsc (51% of double lack of function embryos) (Fig. 6C,C ). This deformation of the prechordal territory points to a slight increase of the CE defect in slb embryos and suggests that Dkk1 may activate the Wnt/PCP pathway. We therefore set out to address this, first, at the biochemical level in Xenopus, in which Dkk1, has been thoroughly studied and biochemical assays are routinely done. We first found that, as in fish, Dkk1 overexpression is inducing CE defects in Xenopus (Fig. 6D G). Animal caps, from embryos injected with activin mrna, are able to form mesoderm and characteristically elongate due to CE movements. After injection of both activin and wnt8, animal cap elongation is slightly enhanced compared with activin alone. Contrasting with this observation, caps injected with activin and dkk1 are unable to elongate, and this is accompanied by induction of gsc at the expense of the mesodermal marker Xbra (Fig. 6M). This loss of elongation is reminiscent of the phenotype induced by gain or loss of Wnt/PCP activity. Indeed, the cells of explants of dorsal blastopore lip (DMZ) in culture normally elongate and orient along the medio-lateral axis (Fig. 6H,L). Cells in explants from Wnt3a-injected embryos show similar or greater orientation relative to the medio-lateral axis (Fig. 6J,L), while dkk1 reduces elongation and loss of orientation relative to the medio-lateral axis (Fig. 6K,L). This cell shape change phenocopies the loss of polarization observed in explants after overexpression of Wnt11 and its receptor Fz7 (Fig. 6I,L). Our data in frog and fish therefore strongly suggest that Dkk1 is able to promote Wnt/PCP activity. To test this possibility at a molecular level, we quantified Wnt/PCP activation by measuring the level of phosphorylation of one of its major transducers, JNK (Boutros et al. 1998). We compared the level of phosphorylated JNK in embryos injected with a HA epitope-tagged JNK mrna alone (control) or together with either wnt11 or dkk1 mrna or both. After specific precipitation of HA-JNK, its level of activation was mea- GENES & DEVELOPMENT 473
10 Caneparo et al. Figure 6. Dkk1 up-regulates the Wnt/PCP pathway. (A C ) Lateral (A C) and dorsal (A B ) views of bud stage wild type (A,A ), slb (B,B ), and slb injected with dkk1mo (C,C ) embryos, expressing hgg (in the hatching gland, hg), ntl (in the notochord, n), and dlx3b (in the neural plate border, nb). (D G) Dkk1 blocks activin-induced animal cap elongation. Xenopus embryos were uninjected (D) or injected animally at the four- to eight-cell stage with activin (E), activin and wnt8 (F), or activin and dkk1. Animal caps were dissected from stage 9 embryos and cultured until sibling embryos had reached stage 17. (H L) Dkk1 disrupts cell polarity. Xenopus embryos were injected at the two- to four-cell stage equatorially with mrfp mrna, and either preprolactin (ctl) or the indicated mrnas (top right corner). Explants of the dorsal upper blastopore lip were cut at stage 10.5, and cells were imaged by confocal microscopy. The yellow line indicates orientation of the medio-lateral axis. (L, left panel) Cell elongation was determined by the mean length-to-width ratio (LWR). (Right panel) Cell orientation was calculated as the percentage of cells with their long axis tilted >20 relative to the medio-lateral axis. (M) RT PCR analysis of animal caps injected as in D G for the indicated genes. (N) Dkk1 activates JNK. HA epitope-tagged JNK mrna was injected either alone or in combination with mrna of the indicated genes into two- to four-cell-stage embryos. HA-JNK was immunoprecipitated from extracts of stage 11 embryos and JNK phosphorylation was monitored using a phospho-specific antibody on Western blot. (ni) Noninjected control. (O R) Dorsal views of bud stage wild-type (O,Q) and mbl (P,R) embryos uninjected (O,P) or injected with 30 pg of dkk1 RNA at the one-cell stage (Q,R), expressing hgg (in the hatching gland, hg), ntl (in the notochord, n), and dlx3b (in the neural plate border, nb). sured by detection of its phosphorylated form, using a phospho-specific antibody. Wnt11 or Dkk1 expression alone is able to enhance JNK phosphorylation, and expression of both of them increases dramatically this phosphorylation, while Wnt8 blocks phosporylation (Fig. 6N). These results demonstrate that Dkk1 directly or indirectly activates the Wnt/PCP pathway. The interaction found between Kny and Dkk1 suggests the possibility of a direct regulation of the Wnt/ PCP pathway. However, it may stimulate Wnt/PCP indirectly since canonical Wnt signaling is able to block the PCP pathway in vitro (Fig. 6N). A direct interaction with the Wnt/PCP is, however, very likely. Indeed, not only is moderate DNwnt8 overexpression unable to rescue the gastrulation movement phenotype in the Dkk1 morphants (Fig. 4J), but we also found that injection of Dkk1 mrna in mbl is inducing a CE defect comparable to the one seen in wild type (Fig. 6O R). This last result therefore shows that Dkk1 can act on CE movements in embryos with a constitutively active Wnt/ catenin pathway. Furthermore, Dkk1 activation of the Wnt/PCP pathway is not due to indirect up-regulation of wnt11 expression, as no increase of wnt11 transcripts is detected after dkk1 RNA injection (Supplementary Fig. S2A C). As Dkk1 is able to up-regulate the PCP pathway, we tested whether it could rescue the CE defect in slb/ wnt11 mutant embryos. Injection of various doses of dkk1 RNA in slb homozygous embryos did not lead to a general rescue (Supplementary Fig. S3). However, high dose of transcripts is able to rescue the shape of the anterior-most axial mesendoderm although not other as- 474 GENES & DEVELOPMENT
11 Dkk1 binds Kny and regulates gastrulation movements pects of the CE defect in slb (Supplementary Fig. S3D E ). This observation shows that Dkk1 is able to partially correct a subset of the cell movement defect induced by loss of Wnt11, namely the rostral progression of the anterior mesendoderm. Dkk1 may act as a switch between the catenin and PCP pathways activated by the Wnt/Fz complex One of our first observations is still unresolved by the results so far. Indeed, the ability of Dkk1 to bind to Kny and activate the Wnt/PCP pathway is not explaining the acceleration of gastrulation movements observed in the Dkk1 morphant embryos. As our data indicate that Dkk1 simultaneously represses the Wnt/ catenin and activates the Wnt/PCP pathway, we tested whether acceleration of gastrulation movements in the Dkk1 morphants may be generated by concomitant up-regulation of the Wnt/ catenin pathway and down-regulation of the Wnt/PCP activity. We injected one- to two-cell-stage donor embryos with either wnt8 RNA, a low dose of dsh- DEP+ (coding for a version of Dishevelled unable to activate the PCP pathway) (Axelrod et al. 1998; Heisenberg et al. 2000), or both transcripts together. We then transplanted cells in the margin of shield stage wildtype host embryos and watched internalization of the donor cells (Fig. 7A D). The cells coming from the double-injected donors do indeed progress faster than the wild-type controls in a majority of the cases (25 of 43) (Fig. 7C,D). Single wnt8-expressing cells display the disperse distribution described above (n = 22) (Fig. 7B), while the DSH-DEP+ cells show difficulty in progression and in CE (n = 25) (Fig. 7A; data not shown). Cells in which the Wnt/ catenin activity is up-regulated and the Wnt/PCP reduced are therefore able to mimic the cellular behavior observed in embryos deprived of Dkk1 activity. In light of all our results, we therefore propose that Dkk1 acts at the level of the Wnt receptors, concomitantly repressing Wnt/ catenin and increasing Wnt/PCP reception, via interaction with both LRP5/6 and Kny (Fig. 7E). Such a model suggests that LRP5/6 and Kny may either compete for binding to Dkk1 or differentially regulate localization of Dkk1. We tested these possibilities by monitoring Dkk1GFP subcellular localization and binding to Kny-Flg in embryos in the presence or absence of exogenous LRP6. We found that, in gastrula embryos expressing both Dkk1GFP and KnyFlg, the distribution of Dkk1GFP is shifted from being found both in cytoplasmic and extracellular location (n = 16; Fig. 7F ) to almost exclusively extracellular (n = 15) (Fig. 7H ). This distribution is completely reversed in embryos that also mildly overexpress LRP6 (1.5 pg of RNA injected at the one-cell stage), where the dkkgfp proteins are mostly found in the cytoplasm (n = 19) (Fig. 7I ). This result shows that Kny alone promotes extracellular localization of Dkk1, while LRP6 drives its endocytosis. We also monitored gastrulation movements in the same four experimental conditions. As expected, injection of dkk1 alone or in combination with kny both induce CE defects. These are more pronounced in the double-injected embryos, highlighting again the cooperation of Dkk1 and Kny in modulation of gastrulation movements (Fig. 7 F,H ). More importantly, mild expression of LRP6 rescues this CE defect (Fig. 7I ). This rescue is not due to direct competition between LRP and Kny for binding to Dkk1. Indeed, our coimmunoprecipitation assay on protein extracts of these embryos (Fig. 7J) shows that LRP6 and Kny do not compete for binding to Dkk1 (Fig. 7J, cf. third and fourth lanes). This finding therefore strongly suggests that Kny is endocytosed with Dkk1 in embryos overexpressing LRP6 and, more importantly, that Dkk1 is able to act concomitantly on both Kny and LRP proteins, thereby modulating both patterning and cell motility. Discussion Dkk1 is a Wnt/ catenin antagonist acting through binding to Kremen and the Frizzled coreceptors LRP5/6 (Mao et al. 2001, 2002). It plays a crucial role in early neural patterning, as its activity is required for maintenance of forebrain identity in the anterior neural plate in both frog and mouse (Mukhopadhyay et al. 2001). Our study uncovered a novel involvement for Dkk1 in modulation of gastrulation movements through binding to the Glypican4-like Knypek and direct regulation of both Wnt/ catenin and PCP signaling pathways. Dkk1 is modulating gastrulation movements When we first observed the changes in cell motility of the internalizing mesendoderm in the Dkk1 morphants, Dkk1 had not yet been associated with regulation of cell movement. Since then, a very recent study has shown that the rostral movement of the AVE just preceding gastrulation in mouse requires Dkk1 (Kimura-Yoshida et al. 2005). Moreover, this study also shows that Wnt/ catenin activity represses such movement and proposes a role of attractant for Dkk1 and repellent for the Wnt ligands. However, when looking at gastrulation movements, the three different approaches taken in our study to up-regulate the Wnt/ catenin pathway in early gastrula embryos all fail to mimic the acceleration in motility observed in the Dkk1 morphants, pointing here to a novel Wnt/ catenin-independent function for Dkk1. Moreover, none of our transplant experiments show cell behavior compatible with an attractant/repellent model. Interestingly, the cell behavior described in the mouse AVE in the presence or absence of Dkk1 activity is also compatible with a fluctuation of the level of both Wnt/ catenin and Wnt/PCP activity, a possibility not tested by the study, as the authors did not question the nature of the pathway modulated by Dkk1. Future analysis of the molecular pathways required for AVE movements in mice is needed to elucidate whether Dkk1 may act on the same set of signaling pathways while regulating the movements of the AVE and those of the mesendoderm. A puzzling observation is that acceleration of gastrulation movements has only been reported once before this study, in zebrafish embryos lacking Lefty function GENES & DEVELOPMENT 475
12 Caneparo et al. Figure 7. Dkk1 may act as a switch between the catenin and PCP pathways activated by the Wnt/Fz complex. (A C) Lateral views of 90% epiboly embryos showing the distribution of the dsh-dep+ (A), wnt8 (B), and dsh-dep + wnt8-expressing cells (blue) transplanted in wild-type hosts at shield stage. The dose injected per donor embryo is 5 pg for dsh-dep+ and 12 pg for wnt8.(d) Seventy-five percent epiboly wild-type host embryo showing the rostral progression of wild-type (brown) and wnt8 + dshdep+ (5 pg + 12 pg, blue) transplanted cells. The dashed line indicates the midline. (E) Model of the proposed mechanism by which Dkk1 may both downregulate the Wnt/ catenin and up-regulate the Wnt/PCP pathways. (F I ) Live embryos injected with 8 pg of dkk1gfp (F,F ), 1.8 pg of kny-flg (G,G ),4pgofdkkGFP pg kny-flg (H,H ),and4pgofdkkgfp + 1.8pg of kny-flg pg of LRP6 (I,I ). Confocal images have been taken at 60% epiboly (F I ) of the GFP localization and the embryos have been left to develop until 48 hpf (F I) to check activity of the transcripts injected. (F I ) Dorsal view, anterior to the top, of embryos injected with dkk1gfp (F ), kny-flg (G ), dkkgfp + kny-flg (H ), and dkkgfp + kny-flg + LRP6 (I ) at the same doses as in F I, showing expression of hgg (in the hatching gland, hg), ntl (in the notochord, n), and dlx3b (in the neural plate border). (J) Western blot of the immunoprecipitation assay done on extracts of 30 late-gastrula injected embryos for each condition tested. Protein extracts from the four combinations of injection (+) are either run untreated (last four lanes) or first immunoprecipitated with an anti-flg antibody (first four lanes), then run on a gel. The gel is then transferred on filter and incubated with an anti-gfp antibody (detecting the GFP-tagged Dkk1 proteins) or an anti-flg antibody (detecting the Flg-tagged Kny proteins. Lanes 3 and 4 show the presence, after Flg immuno-precipitation, of Dkk1-GFP proteins in extracts from embryos expressing both Dkk1GFP and KnyFlag, regardless of LRP6 overexpression. Note that a same set of injected embryos was split into one half for phenotype analysis (F I) and the other for the coimmunoprecipitation assays (J), thereby controlling that the proteins tested for binding were active in the injected embryos. 476 GENES & DEVELOPMENT
Supporting Information
 Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
Xenopus gastrulation. Dorsal-Ventral Patterning - The Spemann Organizer. Two mesoderm inducing signals
 Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
7.22 Final Exam points
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Fertilization. Animal hemisphere. Sperm entry point
 Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Mesoderm Formation. Fate map of early gastrula. Only two types of mesoderm are induced. Mesoderm induction by the vegetal hemisphere
 Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Neural Induction. Chapter One
 Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
7.22 Example Problems for Exam 1 The exam will be of this format. It will consist of 2-3 sets scenarios.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Early Development and Axis Formation in Amphibians
 Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Neural Development. How does a single cell make a brain??? How are different brain regions specified??? Neural Development
 Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
+ + Development and Evolution Dorsoventral axis. Developmental Readout. Foundations. Stem cells. Organ formation.
 Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Lecture 3 MOLECULAR REGULATION OF DEVELOPMENT
 Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Pregnenolone Stabilizes Microtubules and Promotes Zebrafish Embryonic Cell Movement
 Pregnenolone Stabilizes Microtubules and Promotes Zebrafish Embryonic Cell Movement Hwei-Jan Hsu 1,2, Ming-Ren Liang 3, Chao-Tsen Chen 3, and Bon-Chu Chung 1 * Abstract Embryonic cell movement is essential
Pregnenolone Stabilizes Microtubules and Promotes Zebrafish Embryonic Cell Movement Hwei-Jan Hsu 1,2, Ming-Ren Liang 3, Chao-Tsen Chen 3, and Bon-Chu Chung 1 * Abstract Embryonic cell movement is essential
Neural Induction. Steven McLoon Department of Neuroscience University of Minnesota
 Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
SUPPLEMENTARY INFORMATION
 Normalized dorsal trunk length (µm) Normalized dorsal trunk length (TC1-5) (µm) DOI: 1.138/ncb2467 a b c d btl>src64 Src42, btl>src64 e Src64 f g h DT Src64 DT Midgut Src64 Midgut i Src64 Atpα j k l DT
Normalized dorsal trunk length (µm) Normalized dorsal trunk length (TC1-5) (µm) DOI: 1.138/ncb2467 a b c d btl>src64 Src42, btl>src64 e Src64 f g h DT Src64 DT Midgut Src64 Midgut i Src64 Atpα j k l DT
Patterning of the Brain
 Patterning of the Brain Clemens.Kiecker@kcl.ac.uk IoPPN 27 Oct 2015 Hundreds of billions of cells, hundreds of cell types Egg = single cell, some 20,000 genes Egg = single cell, some 20,000 genes How is
Patterning of the Brain Clemens.Kiecker@kcl.ac.uk IoPPN 27 Oct 2015 Hundreds of billions of cells, hundreds of cell types Egg = single cell, some 20,000 genes Egg = single cell, some 20,000 genes How is
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Activity 47.1 What common events occur in the early development of animals? 1. What key events occur at each stage of development?
 Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
Understanding embryonic head development. ANAT2341 Tennille Sibbritt Embryology Unit Children s Medical Research Institute
 Understanding embryonic head development ANAT2341 Tennille Sibbritt Embryology Unit Children s Medical Research Institute Head malformations among the most common category of congenital malformations in
Understanding embryonic head development ANAT2341 Tennille Sibbritt Embryology Unit Children s Medical Research Institute Head malformations among the most common category of congenital malformations in
To assess the localization of Citrine fusion proteins, we performed antibody staining to
 Trinh et al 1 SUPPLEMENTAL MATERIAL FlipTraps recapitulate endogenous protein localization To assess the localization of Citrine fusion proteins, we performed antibody staining to compare the expression
Trinh et al 1 SUPPLEMENTAL MATERIAL FlipTraps recapitulate endogenous protein localization To assess the localization of Citrine fusion proteins, we performed antibody staining to compare the expression
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3363 Supplementary Figure 1 Several WNTs bind to the extracellular domains of PKD1. (a) HEK293T cells were co-transfected with indicated plasmids. Flag-tagged proteins were immunoprecipiated
DOI: 10.1038/ncb3363 Supplementary Figure 1 Several WNTs bind to the extracellular domains of PKD1. (a) HEK293T cells were co-transfected with indicated plasmids. Flag-tagged proteins were immunoprecipiated
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2806 Supplemental Material: Figure S1 Wnt4 PCP phenotypes are not mediated through canonical Wnt signaling. (a-a ) Illustration of wg, dwnt4 and dwnt6 expression patterns in wing (a) and
DOI: 10.1038/ncb2806 Supplemental Material: Figure S1 Wnt4 PCP phenotypes are not mediated through canonical Wnt signaling. (a-a ) Illustration of wg, dwnt4 and dwnt6 expression patterns in wing (a) and
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development
 Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5
 Eddy De Robertis Page 1 MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5 Having discussed the early events in mesoderm induction, we now turn to signaling events that take place
Eddy De Robertis Page 1 MOLECULAR BIOLOGY OF SPEMANN S ORGANIZER AND NEURAL INDUCTION - Lecture 5 Having discussed the early events in mesoderm induction, we now turn to signaling events that take place
Stargazin regulates AMPA receptor trafficking through adaptor protein. complexes during long term depression
 Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
7.012 Problem Set 5. Question 1
 Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth
 RESEARCH ARTICLE 1071 Development 136, 1071-1081 (2009) doi:10.1242/dev.032912 The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth Amanda
RESEARCH ARTICLE 1071 Development 136, 1071-1081 (2009) doi:10.1242/dev.032912 The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth Amanda
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Hipk2 and PP1c Cooperate to Maintain Dvl Protein Levels Required for Wnt Signal Transduction
 Cell Reports Article Hipk2 and PP1c Cooperate to Maintain Dvl Protein Levels Required for Wnt Signal Transduction Nobuyuki Shimizu, 1 Shizuka Ishitani, 1 Atsushi Sato, 2 Hiroshi Shibuya, 2 and Tohru Ishitani
Cell Reports Article Hipk2 and PP1c Cooperate to Maintain Dvl Protein Levels Required for Wnt Signal Transduction Nobuyuki Shimizu, 1 Shizuka Ishitani, 1 Atsushi Sato, 2 Hiroshi Shibuya, 2 and Tohru Ishitani
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
(a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for
 Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
Supplementary Figure 1. Wnt10a mutation causes aberrant molar tooth development and formation of an ectopic M4 molar. (a,b) Smaller molar teeth with
 Supplementary Figure 1. Wnt10a mutation causes aberrant molar tooth development and formation of an ectopic M4 molar. (a,b) Smaller molar teeth with blunted cusp formation, and presence of an ectopic molar
Supplementary Figure 1. Wnt10a mutation causes aberrant molar tooth development and formation of an ectopic M4 molar. (a,b) Smaller molar teeth with blunted cusp formation, and presence of an ectopic molar
The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways
 Developmental Biology 305 (2007) 103 119 www.elsevier.com/locate/ydbio The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways Bo
Developmental Biology 305 (2007) 103 119 www.elsevier.com/locate/ydbio The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways Bo
Induction and patterning of the telencephalon in Xenopus laevis
 Development 129, 5421-5436 (2002) 2002 The Company of Biologists Ltd doi:10.1242/dev.00095 5421 Induction and patterning of the telencephalon in Xenopus laevis Giuseppe Lupo 1,2, William A. Harris 2, Giuseppina
Development 129, 5421-5436 (2002) 2002 The Company of Biologists Ltd doi:10.1242/dev.00095 5421 Induction and patterning of the telencephalon in Xenopus laevis Giuseppe Lupo 1,2, William A. Harris 2, Giuseppina
Supplementary Information. Isl2b regulates anterior second heart field development in zebrafish
 Supplementary Information Isl2b regulates anterior second heart field development in zebrafish Hagen R. Witzel 1, Sirisha Cheedipudi 1, Rui Gao 1, Didier Y.R. Stainier 2 and Gergana D. Dobreva 1,3* 1 Origin
Supplementary Information Isl2b regulates anterior second heart field development in zebrafish Hagen R. Witzel 1, Sirisha Cheedipudi 1, Rui Gao 1, Didier Y.R. Stainier 2 and Gergana D. Dobreva 1,3* 1 Origin
Supplementary Data Supplementary Figures
 Supplementary Data Supplementary Figures Supplementary Figure 1. Pi04314 is expressed during infection, each GFP-Pi04314 fusion is stable and myr GFP-Pi04314 is removed from the nucleus while NLS GFP-Pi04314
Supplementary Data Supplementary Figures Supplementary Figure 1. Pi04314 is expressed during infection, each GFP-Pi04314 fusion is stable and myr GFP-Pi04314 is removed from the nucleus while NLS GFP-Pi04314
TRANSGENIC ANIMALS. -transient transfection of cells -stable transfection of cells. - Two methods to produce transgenic animals:
 TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplemental Information
 Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Figure S2. Verification of ihog and boi
 Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
monoclonal antibody. (a) The specificity of the anti-rhbdd1 monoclonal antibody was examined in
 Supplementary information Supplementary figures Supplementary Figure 1 Determination of the s pecificity of in-house anti-rhbdd1 mouse monoclonal antibody. (a) The specificity of the anti-rhbdd1 monoclonal
Supplementary information Supplementary figures Supplementary Figure 1 Determination of the s pecificity of in-house anti-rhbdd1 mouse monoclonal antibody. (a) The specificity of the anti-rhbdd1 monoclonal
Supplementary Figure 1 A
 Supplementary Figure A B M. mullata p53, 3 UTR Luciferase activity (%) mir-5b 8 Le et al. Supplementary Information NC-DP - + - - - - - NC-DP - - + - - - - NC-DP3 - - - + - - - 5b-DP - - - - + + + NC-AS
Supplementary Figure A B M. mullata p53, 3 UTR Luciferase activity (%) mir-5b 8 Le et al. Supplementary Information NC-DP - + - - - - - NC-DP - - + - - - - NC-DP3 - - - + - - - 5b-DP - - - - + + + NC-AS
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Heparanase Modulates Chondrogenic Factor Signaling And Is Upregulated In Ectopic Cartilage
 Heparanase Modulates Chondrogenic Factor Signaling And Is Upregulated In Ectopic Cartilage Julianne Huegel, Motomi Enomoto-Iwamoto, PhD, Federica Sgariglia, Tricia Bhatti, Eiki Koyama, Maurizio Pacifici.
Heparanase Modulates Chondrogenic Factor Signaling And Is Upregulated In Ectopic Cartilage Julianne Huegel, Motomi Enomoto-Iwamoto, PhD, Federica Sgariglia, Tricia Bhatti, Eiki Koyama, Maurizio Pacifici.
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or
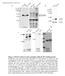 Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated
 Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
Nature Biotechnology: doi: /nbt Supplementary Figure 1
 Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Kunduri et al., http://www.jcb.org/cgi/content/full/jcb.201405020/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Subcellular localization and interactions of PAPLA1.
Supplemental material Kunduri et al., http://www.jcb.org/cgi/content/full/jcb.201405020/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Subcellular localization and interactions of PAPLA1.
MATERIALS AND METHODS
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 19, pp. 13247 13257, May 12, 2006 2006 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Mouse Cristin/R-spondin
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 19, pp. 13247 13257, May 12, 2006 2006 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Mouse Cristin/R-spondin
Supplementary Figure 1. Drawing of spinal cord open-book preparations and DiI tracing. Nature Neuroscience: doi: /nn.3893
 Supplementary Figure 1 Drawing of spinal cord open-book preparations and DiI tracing. Supplementary Figure 2 In ovo electroporation of dominant-negative PlexinA1 in commissural neurons induces midline
Supplementary Figure 1 Drawing of spinal cord open-book preparations and DiI tracing. Supplementary Figure 2 In ovo electroporation of dominant-negative PlexinA1 in commissural neurons induces midline
Sperm cells are passive cargo of the pollen tube in plant fertilization
 In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17079 Sperm cells are passive cargo of the pollen tube in plant fertilization Jun Zhang 1, Qingpei
In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17079 Sperm cells are passive cargo of the pollen tube in plant fertilization Jun Zhang 1, Qingpei
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary figure 1: List of primers/oligonucleotides used in this study. 1 Supplementary figure 2: Sequences and mirna-targets of i) mcherry expresses in transgenic fish used
SUPPLEMENTARY INFORMATION Supplementary figure 1: List of primers/oligonucleotides used in this study. 1 Supplementary figure 2: Sequences and mirna-targets of i) mcherry expresses in transgenic fish used
embryos. Asterisk represents loss of or reduced expression. Brackets represent
 Supplemental Figures Supplemental Figure 1. tfec expression is highly enriched in tail endothelial cells (A- B) ISH of tfec at 15 and 16hpf in WT embryos. (C- D) ISH of tfec at 36 and 38hpf in WT embryos.
Supplemental Figures Supplemental Figure 1. tfec expression is highly enriched in tail endothelial cells (A- B) ISH of tfec at 15 and 16hpf in WT embryos. (C- D) ISH of tfec at 36 and 38hpf in WT embryos.
Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3
 The EMBO Journal Vol.17 No.12 pp.3413 3427, 1998 Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3 Harry V.Isaacs 1, Mary Elizabeth Pownall and Jonathan
The EMBO Journal Vol.17 No.12 pp.3413 3427, 1998 Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3 Harry V.Isaacs 1, Mary Elizabeth Pownall and Jonathan
Supplementary Figure 1 Collision-induced dissociation (CID) mass spectra of peptides from PPK1, PPK2, PPK3 and PPK4 respectively.
 Supplementary Figure 1 lision-induced dissociation (CID) mass spectra of peptides from PPK1, PPK, PPK3 and PPK respectively. % of nuclei with signal / field a 5 c ppif3:gus pppk1:gus 0 35 30 5 0 15 10
Supplementary Figure 1 lision-induced dissociation (CID) mass spectra of peptides from PPK1, PPK, PPK3 and PPK respectively. % of nuclei with signal / field a 5 c ppif3:gus pppk1:gus 0 35 30 5 0 15 10
PHT1;2-CFP YFP-PHF + PHT1;2-CFP YFP-PHF
 YFP-PHF1 CFP-PHT1;2 PHT1;2-CFP YFP-PHF + PHT1;2-CFP YFP-PHF + CFP-PHT1;2 Negative control!-gfp Supplemental Figure 1: PHT1;2 accumulation is PHF1 dependent. Immunoblot analysis on total protein extract
YFP-PHF1 CFP-PHT1;2 PHT1;2-CFP YFP-PHF + PHT1;2-CFP YFP-PHF + CFP-PHT1;2 Negative control!-gfp Supplemental Figure 1: PHT1;2 accumulation is PHF1 dependent. Immunoblot analysis on total protein extract
Biology Open (2015) 000, 1 16 doi: /bio
 Supplementary Material Annekatrin König and Halyna R. Shcherbata doi: 10.1242/ bio.201410553 Fig. S1. Histones are differentially modified in somatic and germline cells in the germarium. Wildtype (OregonR)
Supplementary Material Annekatrin König and Halyna R. Shcherbata doi: 10.1242/ bio.201410553 Fig. S1. Histones are differentially modified in somatic and germline cells in the germarium. Wildtype (OregonR)
1. Supplementary Tables
 SUPPLEMENTARY INFORMATION doi:10.1038/nature14215 1. Supplementary Tables Supplementary Table 1 Phenotype of medaka YAP MO-injected embryos Morpholinos Host Amount of MOs Human YAP mrna Total survived
SUPPLEMENTARY INFORMATION doi:10.1038/nature14215 1. Supplementary Tables Supplementary Table 1 Phenotype of medaka YAP MO-injected embryos Morpholinos Host Amount of MOs Human YAP mrna Total survived
7.013 Practice Quiz
 MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
1. 40 points Retinoic acid (structure below) is a small hydrophobic molecule that is derived from vitamin A. Figure by MIT OCW.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 9 7.22 Fall 2005 exam 2 practice
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 9 7.22 Fall 2005 exam 2 practice
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of
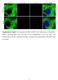 Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Supplemental Data. Cui et al. (2012). Plant Cell /tpc a b c d. Stem UBC32 ACTIN
 A Root Stem Leaf Flower Silique Senescence leaf B a b c d UBC32 ACTIN C * Supplemental Figure 1. Expression Pattern and Protein Sequence of UBC32 Homologues in Yeast, Human, and Arabidopsis. (A) Expression
A Root Stem Leaf Flower Silique Senescence leaf B a b c d UBC32 ACTIN C * Supplemental Figure 1. Expression Pattern and Protein Sequence of UBC32 Homologues in Yeast, Human, and Arabidopsis. (A) Expression
Trasposable elements: Uses of P elements Problem set B at the end
 Trasposable elements: Uses of P elements Problem set B at the end P-elements have revolutionized the way Drosophila geneticists conduct their research. Here, we will discuss just a few of the approaches
Trasposable elements: Uses of P elements Problem set B at the end P-elements have revolutionized the way Drosophila geneticists conduct their research. Here, we will discuss just a few of the approaches
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Kimura et al.,
 Supplemental material JCB Kimura et al., http://www.jcb.org/cgi/content/full/jcb.201503023/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. TRIMs regulate IFN-γ induced autophagy. (A and B) HC image analysis
Supplemental material JCB Kimura et al., http://www.jcb.org/cgi/content/full/jcb.201503023/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. TRIMs regulate IFN-γ induced autophagy. (A and B) HC image analysis
Later Development. Caenorhabditis elegans. Later Processes 10/06/12. Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development
 Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Conserved Patterns of Cell Movements during Vertebrate Gastrulation. Review. Lilianna Solnica-Krezel
 Current Biology, Vol. 15, R213 R228, March 29, 2005, 2005 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2005.03.016 Conserved Patterns of Cell Movements during Vertebrate Gastrulation Review Lilianna
Current Biology, Vol. 15, R213 R228, March 29, 2005, 2005 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2005.03.016 Conserved Patterns of Cell Movements during Vertebrate Gastrulation Review Lilianna
Supplementary Figures
 1 Supplementary Figures Supplementary Figure 1 Dynamics of DCL cell division and its relation to cell dispersion during epiboly of A. nigripinnis. (a) Temporal changes in the cumulative number of DCL cell
1 Supplementary Figures Supplementary Figure 1 Dynamics of DCL cell division and its relation to cell dispersion during epiboly of A. nigripinnis. (a) Temporal changes in the cumulative number of DCL cell
Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product.
 Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
Practice Problems 5. Location of LSA-GFP fluorescence
 Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Revision Checklist for Science Signaling Research Manuscripts: Data Requirements and Style Guidelines
 Revision Checklist for Science Signaling Research Manuscripts: Data Requirements and Style Guidelines Further information can be found at: http://stke.sciencemag.org/sites/default/files/researcharticlerevmsinstructions_0.pdf.
Revision Checklist for Science Signaling Research Manuscripts: Data Requirements and Style Guidelines Further information can be found at: http://stke.sciencemag.org/sites/default/files/researcharticlerevmsinstructions_0.pdf.
A requirement for NF-protocadherin and TAF1/Set in cell adhesion and neural tube formation
 Developmental Biology 291 (2006) 170 181 www.elsevier.com/locate/ydbio A requirement for NF-protocadherin and TAF1/Set in cell adhesion and neural tube formation Dana Rashid, Katie Newell, Leah Shama,
Developmental Biology 291 (2006) 170 181 www.elsevier.com/locate/ydbio A requirement for NF-protocadherin and TAF1/Set in cell adhesion and neural tube formation Dana Rashid, Katie Newell, Leah Shama,
Supplementary Figure 1. Isolation of GFPHigh cells.
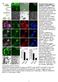 Supplementary Figure 1. Isolation of GFP High cells. (A) Schematic diagram of cell isolation based on Wnt signaling activity. Colorectal cancer (CRC) cell lines were stably transduced with lentivirus encoding
Supplementary Figure 1. Isolation of GFP High cells. (A) Schematic diagram of cell isolation based on Wnt signaling activity. Colorectal cancer (CRC) cell lines were stably transduced with lentivirus encoding
Nature Structural & Molecular Biology: doi: /nsmb.1583
 Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
(phosphatase tensin) domain is shown in dark gray, the FH1 domain in black, and the
 Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Charles Shuler, Ph.D. University of Southern California Los Angeles, California Approved for Public Release; Distribution Unlimited
 AD Award Number: DAMD17-01-1-0100 TITLE: Smad-Mediated Signaling During Prostate Growth and Development PRINCIPAL INVESTIGATOR: Charles Shuler, Ph.D. CONTRACTING ORGANIZATION: University of Southern California
AD Award Number: DAMD17-01-1-0100 TITLE: Smad-Mediated Signaling During Prostate Growth and Development PRINCIPAL INVESTIGATOR: Charles Shuler, Ph.D. CONTRACTING ORGANIZATION: University of Southern California
Bi8 Lecture 19. Review and Practice Questions March 8th 2016
 Bi8 Lecture 19 Review and Practice Questions March 8th 2016 Common Misconceptions Where Words Matter Common Misconceptions DNA vs RNA vs Protein Su(H) vs Su(H) Replication Transcription Transcribed vs
Bi8 Lecture 19 Review and Practice Questions March 8th 2016 Common Misconceptions Where Words Matter Common Misconceptions DNA vs RNA vs Protein Su(H) vs Su(H) Replication Transcription Transcribed vs
Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mot
 Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mottershead* Open Access Address: Programme for Developmental
Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mottershead* Open Access Address: Programme for Developmental
Biphasic wnt8a Expression Is Achieved Through Interactions of Multiple Regulatory Inputs
 a DEVELOPMENTAL DYNAMICS 241:1062 1075, 2012 RESEARCH ARTICLE Biphasic wnt8a Expression Is Achieved Through Interactions of Multiple Regulatory Inputs Anand Narayanan and Arne C. Lekven* Background: Vertebrate
a DEVELOPMENTAL DYNAMICS 241:1062 1075, 2012 RESEARCH ARTICLE Biphasic wnt8a Expression Is Achieved Through Interactions of Multiple Regulatory Inputs Anand Narayanan and Arne C. Lekven* Background: Vertebrate
Supplemental Information. Pacer Mediates the Function of Class III PI3K. and HOPS Complexes in Autophagosome. Maturation by Engaging Stx17
 Molecular Cell, Volume 65 Supplemental Information Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17 Xiawei Cheng, Xiuling Ma, Xianming Ding,
Molecular Cell, Volume 65 Supplemental Information Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17 Xiawei Cheng, Xiuling Ma, Xianming Ding,
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
c) Assuming he does not run another endurance race, will the steady-state populations be affected one year later? If so, explain how.
 LS1a Fall 06 Problem Set #8 (100 points total) all questions including the (*extra*) one should be turned in 1. (18 points) Erythrocytes, mature red blood cells, are essential for transporting oxygen to
LS1a Fall 06 Problem Set #8 (100 points total) all questions including the (*extra*) one should be turned in 1. (18 points) Erythrocytes, mature red blood cells, are essential for transporting oxygen to
Optimizing Cas9 and sgrna concentrations for increasing mutation efficiency. Nature Methods: doi: /nmeth.3360
 Supplementary Figure 1 Optimizing Cas9 and sgrna concentrations for increasing mutation efficiency. (a) Wild-type, 2-dpf slc24a5 b1 embryos and a series of slc24a5 CRISPR-injected embryos; anterior is
Supplementary Figure 1 Optimizing Cas9 and sgrna concentrations for increasing mutation efficiency. (a) Wild-type, 2-dpf slc24a5 b1 embryos and a series of slc24a5 CRISPR-injected embryos; anterior is
T H E J O U R N A L O F C E L L B I O L O G Y
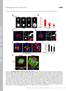 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling
 Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling Go-Woon Kim, Jong-Hoon Won, Ok-Kyung Lee, Sang-Soo Lee, Jeong-Hoon Han, Orkhon Tsogtbaatar, Sujin Nam, Yeon Kim, and Kyung-Ok Cho* Supplementary
Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling Go-Woon Kim, Jong-Hoon Won, Ok-Kyung Lee, Sang-Soo Lee, Jeong-Hoon Han, Orkhon Tsogtbaatar, Sujin Nam, Yeon Kim, and Kyung-Ok Cho* Supplementary
Supplementary information
 Supplementary information Supplementary figures Figure S1 Level of mycdet1 protein in DET1 OE-1, OE-2 and OE-3 transgenic lines. Total protein extract from wild type Col0, det1-1 mutant and DET1 OE lines
Supplementary information Supplementary figures Figure S1 Level of mycdet1 protein in DET1 OE-1, OE-2 and OE-3 transgenic lines. Total protein extract from wild type Col0, det1-1 mutant and DET1 OE lines
HD1 - Introduction to Human Development
 Human Development Ed Laufer, PhD Course Director elaufer@columbia.edu Ping Feng Course Administrator pf2013@columbia.edu Michael Shen, PhD Patricia Ducy, PhD Michael Gershon, MD Cathy Mendelsohn, PhD Richard
Human Development Ed Laufer, PhD Course Director elaufer@columbia.edu Ping Feng Course Administrator pf2013@columbia.edu Michael Shen, PhD Patricia Ducy, PhD Michael Gershon, MD Cathy Mendelsohn, PhD Richard
Regulation of distinct branches of the non-canonical Wnt-signaling network in Xenopus dorsal marginal zone explants
 Wallkamm et al. BMC Biology (2016) 14:55 DOI 10.1186/s12915-016-0278-x RESEARCH ARTICLE Open Access Regulation of distinct branches of the non-canonical Wnt-signaling network in Xenopus dorsal marginal
Wallkamm et al. BMC Biology (2016) 14:55 DOI 10.1186/s12915-016-0278-x RESEARCH ARTICLE Open Access Regulation of distinct branches of the non-canonical Wnt-signaling network in Xenopus dorsal marginal
Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis
 Development 130, 2129-2138 2003 The Company of Biologists Ltd doi:10.1242/dev.00435 2129 Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis Bisei
Development 130, 2129-2138 2003 The Company of Biologists Ltd doi:10.1242/dev.00435 2129 Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis Bisei
Supplementary Figure S1. N-terminal fragments of LRRK1 bind to Grb2.
 Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Supplementary Figure 1. α-synuclein is truncated in PD and LBD brains. Nature Structural & Molecular Biology: doi: /nsmb.
 Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Mouse Cristin/R-spondin family proteins are novel ligands for the Frizzled8 and LRP6 receptors and activate -catenin-dependent gene expression
 JBC Papers in Press. Published on March 16, 2006 as Manuscript M508324200 The latest version is at http://www.jbc.org/cgi/doi/10.1074/jbc.m508324200 Mouse Cristin/R-spondin family proteins are novel ligands
JBC Papers in Press. Published on March 16, 2006 as Manuscript M508324200 The latest version is at http://www.jbc.org/cgi/doi/10.1074/jbc.m508324200 Mouse Cristin/R-spondin family proteins are novel ligands
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Concepts and Methods in Developmental Biology
 Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
LIFE Formation III. Morphogenesis: building 3D structures START FUTURE. How-to 2 PROBLEMS FORMATION SYSTEMS SYSTEMS.
 7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
Supplemental Figure 1 (Figure S1), related to Figure 1 Figure S1 provides evidence to demonstrate Nfatc1Cre is a mouse line that directed gene
 Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
