Numb Controls Integrin Endocytosis for Directional Cell Migration with apkc and PAR-3
|
|
|
- Polly Mason
- 6 years ago
- Views:
Transcription
1 Article Numb Controls Integrin Endocytosis for Directional Cell Migration with apkc and PAR-3 Takashi Nishimura 1,2 and Kozo Kaibuchi 1, * 1 Department of Cell Pharmacology, Graduate School of Medicine, Nagoya University, 65 Tsurumai, Showa, Nagoya, Aichi , Japan 2 Present address: IMBA-Institute of Molecular Biotechnology, Dr. Bohr-Gasse 3, 1030 Vienna, Austria. *Correspondence: kaibuchi@med.nagoya-u.ac.jp DOI /j.devcel SUMMARY Migrating cells extend protrusions to establish new adhesion sites at their leading edges. One of the driving forces for cell migration is the directional trafficking of cell-adhesion molecules such as integrins. Here, we show that the endocytic adaptor protein Numb is an important component of the machinery for directional integrin trafficking in migrating cells. Numb binds to integrin-bs and localizes to clathrin-coated structures (CCSs) at the substratum-facing surface of the leading edge. Numb inhibition by RNAi impairs both integrin endocytosis and cell migration toward integrin substrates. Numb is regulated by phosphorylation since the protein is released from CCSs and no longer binds integrins when phosphorylated by atypical protein kinase C (apkc). Because Numb interacts with the apkc binding partner PAR-3, we propose a model in which polarized Numb phosphorylation contributes to cell migration by directing integrin endocytosis to the leading edge. INTRODUCTION Integrins are major cell-adhesion receptors and play key roles in development, immune response, and cancer metastasis. These heterodimeric transmembrane receptors, composed of an a and a b subunit, bind to the extracellular matrix and initiate intracellular signaling events that regulate cell migration (Webb et al., 2002; Ridley et al., 2003). Focal adhesions are multiple assemblies of proteins that form at the sites of clustered integrin adhesion. The components of focal adhesions, such as talin, vinculin, focal adhesion kinase (FAK), and paxillin, anchor bundles of actin stress fibers and convey signals intracellularly. Focal-adhesion formation at the cell front and disassembly at the rear are critical for the migration of many adherent cells. The assembly of focal adhesions is driven by Rho GTPase and integrin engagement (Kaibuchi et al., 1999; Webb et al., 2002). In contrast, calpain-mediated cleavage of talin controls focal-adhesion disassembly (Franco et al., 2004). It has been proposed that integrins are recycled from the rear of a cell to its front via endocytosis (Bretscher and Aguado-Velasco, 1998). A part of internalized integrin was shown to be recycled via Rab4- dependent early recycling endosomes or Rab11-dependent late recycling endosomes (Roberts et al., 2001; Jones et al., 2006). Indeed, recycling of integrins plays a role in cell migration in several cells, and recent papers have provided mechanistic insight into how integrin traffic is regulated (Jones et al., 2006; Pellinen and Ivaska, 2006). However, the molecular mechanisms for how integrin is internalized at the specialized cell area are largely unknown. Numb directly binds to several endocytic proteins, including a-adaptin and Eps15 (Salcini et al., 1997; Santolini et al., 2000). Numb is thought to be a PTB domain containing a cargo-specific adaptor protein, which selectively binds to certain transmembrane receptors and links them to the endocytic machinery for internalization of receptors (Traub, 2003; Sorkin, 2004). Numb was originally implicated in the cell-fate determination of peripheral and central nervous system development in Drosophila (Betschinger and Knoblich, 2004; Roegiers and Jan, 2004). During neuroblast and sensory organ precursor cell division, Numb localizes asymmetrically and segregates into the one daughter cell. Numb may influence cell fate by downregulating Notch signaling through polarized endocytosis of Notch, because Numb binds to the intracellular domain of Notch (Berdnik et al., 2002). We previously reported that Numb localizes to the tip of growing axons in cultured hippocampal neurons and plays a role in the internalization of L1, a neuronal cell-adhesion molecule, for axon growth (Nishimura et al., 2003). Internalized L1 is transported to the leading edge of the growth cones by microtubule-dependent transport and is inserted into the plasma membrane to form new adhesions (Kamiguchi and Lemmon, 2000). We here report the role of Numb in integrin endocytosis for directional cell migration. In addition, a multifunctional PAR polarity complex regulates the localization and function of Numb. Characterizing the localization and function of Numb as a cargo-selective adaptor in migrating cells provides the basis for further understanding of cell migration and recycling of cell-adhesion molecules. Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc. 15
2 Figure 1. Numb Localizes to Clathrin-Coated Structures at the Substratum-Facing Surface (A) Numb preferentially localizes to clathrin-coated structures (CCSs) at the substratum-facing surface. HeLa cells were fixed shortly after wounding and were stained with the indicated antibodies and phalloidin to visualize F-actin. The enlarged images in the boxed area and the x-z images along with the line in the picture are shown (bottom). (B) Numb polarizes toward the leading edge and the rear in ECV304 cells (as indicated by arrows). The scale bars are 10 mm. (C) Specificity of the anti-numb antibody. Asterisks indicate the Numb-suppressed HeLa cells by sirna. (D) Localization of Numb to CCSs is dependent on its DPF motif in Vero cells. The scale bar is 5 mm. (E) Domain structure and mutants of Numb. D/N, DLA/NLA mutant. Circles, normal localization to CCSs; triangles, weak localization; crosses, undetectable localization. (F) Knockdown of Numb, AP-2, and clathrin in HeLa cells by sirna. (G) Localization of Numb to CCSs is dependent on the AP-2 complex in HeLa or Vero cells. Essentially similar results were observed in both cell types. Data are from Vero cells (sinumb) or HeLa cells (siap-2). Asterisks indicate the depleted cells by each sirna. The scale bar is 10 mm. RESULTS Localization of Numb to Clathrin-Coated Structures We first examined the subcellular localization of Numb in several cell lines. Numb preferentially localized to the AP-2-positive clathrin-coated structures (CCSs) at the substratum-facing surface in HeLa epithelial-like cells (Figure 1A). However, Numb did not always localize to all of the AP-2-positive CCSs at the basal surface. The puncta of Numb were polarized toward the leading edge and sometimes, but not always, toward the rear in migrating ECV304 endothelial cells (Figure 1B), Vero fibroblasts, and HeLa cells (see below). Numb has been reported to localize not only to CCSs at the plasma membrane, but also to early endosomes, late endosomes, and trans-golgi networks (Santolini et al., 2000). However, Numb puncta were colocalized neither with EEA1, an early endosome marker (Figure S3C; see the Supplemental Data available with this article online), nor g-adaptin, a trans-golgi network marker (data not shown). The punctate staining of Numb at the substratum-facing surface was strongly reduced upon knockdown of the protein by Numb sirna (Figures 1C and 1F; Figure S1A), indicating that Numb staining is specific. In addition, we detected Numb immunostaining in the nucleus, which did not disappear upon Numb knockdown and is therefore considered nonspecific. We confirmed these observations by using a different anti-numb antibody (Figure S1D). Consistent with the immunostaining, we found that GFPfused, full-length Numb preferentially localized to the AP- 2-positive CCSs at the substratum-facing surface (Figure 1D; Figures S2A and S2D). To test which region of Numb is important for the localization, we used several Numb mutants. The C-terminal part of Numb (Numb-PRR) 16 Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc.
3 Figure 2. Numb Polarizes toward the Leading Edge and Localizes around Focal Adhesions (A) Numb polarizes toward the wound edge. Three hours after wounding, HeLa cells were fixed and stained. Dashed lines indicate the leading edge, as judged by phallioidin staining. (B) Quantitative analysis for the localization of Numb and a-adaptin in directionally migrating HeLa cells after wounding. (C) Numb localizes around focal adhesions in directionally migrating cells. Four hours after wounding, HeLa cells were fixed and stained. (D) Quantitative analysis for the distribution of Numb and a-adaptin in adhesion and nonadhesion areas. The distribution of Numb and a-adaptin in each area was judged by vinculin or GFP-zyxin as adhesion markers. (E) Numb localizes around focal adhesions. HeLa cells were fixed 1 hr after replating on fibronectin (FN)- or collagen (Col)-coated coverslips or 2 hr after incubation with 0.1 mm cytochalasin-d (CCD) or 10 mm nocodazole (Noco) in the absence of serum. (F) Numb preferentially accumulates around focal adhesions as compared with CCSs. HeLa cells stably expressing GFP-zyxin or GFP-clathrin-lightchain (CLC) were fixed 1 hr after replating on collagen-coated coverslips. The scale bars are 10 mm. weakly localized to CCSs, whereas the N-terminal part of Numb (Numb-PTB) did not (data not shown; Figure 1E). Mutations in the a-adaptin-binding DPF motif (Numbfull-DLA) strongly reduced the localization to CCSs, whereas those in the Eps15-binding NPF motif (Numbfull-NLA) did not (Figures 1D and 1E; Figure S2B). Thus, the a-adaptin-binding DPF motif is required for the localization to CCSs, and the N-terminal part of Numb may control its localization. In addition, the punctate staining of Numb disappeared by suppression of the AP-2 complex by m2-adaptin sirna (Figures 1F and 1G). In contrast, the suppression of clathrin-heavy-chain did not affect the localization of Numb (Figure S2C) and AP-2 (Motley et al., 2003). The punctate staining of a- adaptin and clathrin-heavy-chain (data not shown) was not affected by the suppression of Numb. These results indicate that the AP-2 complex is required for the localization of Numb to CCSs. Numb Polarizes toward the Leading Edge and Localizes around Focal Adhesions We characterized the localization of Numb in more detail in migrating HeLa cells. HeLa cells directionally migrated into the wounding area in an in vitro wound-healing assay (see Figure 4G). Numb accumulated at the cell front just inside the leading edge. To quantify the Numb localization, we compared the localization of Numb and a-adaptin simultaneously at the substratum-facing surface shortly after and 3 hr after wounding (Figures 1A and 2A). Quantitative analysis revealed that the distribution of a-adaptinpositive CCSs was polarized toward the leading edge (Figure 2B), as reported in migrating MDCKII cells (Rappoport and Simon, 2003). However, Numb was more markedly distributed near the leading edge among a-adaptinpositive CCSs after wounding. Thus, Numb localization becomes polarized coincidently with directional migration, which is not reflected by the global change of the Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc. 17
4 distribution of CCSs at the substratum-facing surface. A similar distribution of Numb was observed in migrating ECV304 cells and Vero cells (Figure 1B; Figures S3A S3D). Migrating cells form many adhesion sites at the cell front to facilitate the migration. However, AP-2-positive CCSs almost uniformly distribute at the substratum-facing surface irrespective of the position of adhesions (see below). Thus, we investigated the distribution of Numbpositive CCSs and adhesion sites. As expected, Numb did not colocalize with vinculin-positive adhesions at the substratum-facing surface (Figure 2C). Numb preferentially localized around adhesion sites in directionally migrating HeLa cells after wounding. This observation was confirmed by measuring the fluorescence intensity of Numb at the adhesion area and the adjacent nonadhesion area (Figure 2D). To further examine whether Numb localizes around adhesions, we replated HeLa cells on fibronectin- or collagen-coated coverslips to activate integrin adhesions. Numb accumulated around vinculinpositive focal adhesions at the cell peripheral region 1 hr after plating (Figures 2D and 2E). To directly compare the distribution of Numb and CCSs relative to that of adhesions, we established HeLa cell lines stably expressing GFP-zyxin or GFP-clathrin-light-chain as a marker. Numb was markedly distributed in adhesion areas labeled by GFP-zyxin or endogenous vinculin, whereas endogenous a-adaptin or GFP-clathrin-light-chain were nearly equally distributed in both areas at the substratum-facing surface (Figures 2D and 2F). These results indicate that Numb preferentially localizes around focal adhesions among CCSs. To further investigate Numb localization, we examined the contribution of actin and the microtubule cytoskeleton. When resting HeLa cells were treated with nocodazole to depolymerize microtubules, cells formed enlarged adhesions. Under these conditions, there was a marked accumulation of Numb around large focal adhesions (Figures 2D and 2E; Figure S4A). However, when adhesions were reduced upon partial disruption of F-actin by cytochalasin-d, Numb randomly distributed to a-adaptin-positive CCSs irrespective of the position of the remaining small adhesions. These results suggest that F-actin and/or the adhesion itself, but not microtubules, are important for the preferential localization of Numb around adhesions. However, Numb still localized at the basal surface under both conditions (data not shown). Numb Binds to Integrin and Functions in Integrin Endocytosis Localization of Numb around focal adhesions at the substratum-facing surface prompted us to examine whether Numb interacts with integrin and controls integrin endocytosis. Some Numb-positive puncta (10% of the total) colocalized with cell-surface integrin-b1 at the substratum-facing surface in directionally migrating HeLa cells (Figure 3A). GST-Numb-PTB precipitated integrin-b1 and integrin-b3 from rat brain lysate, whereas GST-Numb-PRR did not (Figure 3B). Similar results were obtained from HeLa cell lysate (data not shown). The PTB domain of Numb has been shown to consistently and directly bind to the integrin-b cytoplasmic region (Calderwood et al., 2003). However, Numb did not bind the components of focal adhesions, such as talin, FAK, vinculin, paxillin, and a-actinin. GST-Numb-full weakly interacted with integrin-b1 and integrin-b3 compared to GST-Numb-PTB, suggesting that the interaction may be regulated by its C-terminal part. In addition, Numb weakly immunoprecipitated integrin-b1 from HeLa cells (Figure 3C). These results raise the possibility that Numb binds to integrin in vivo and functions in integrin endocytosis. To examine the implication of Numb in integrin endocytosis, we next visualized the internalized integrin-b in directionally migrating HeLa cells. Endocytic compartments labeled with the anti-integrin-b1 antibody showed the punctate localization of internalized integrin-b1. The suppression of Numb resulted in the reduction of internalized integrin-b1-positive vesicles (Figures 4A and 4B). We further examined the effects of Numb knockdown in rounded HeLa cells after reseeding on poly-d-lysin (PDL) to simplify the internalization assay. Numb knockdown inhibited both integrin-b1 and integrin-b3 endocytosis, whereas it did not inhibit transferrin endocytosis (Figures 4C and 4D; Figure S4B). For rescue experiments, we produced sirna-resistant (sr) Numb mutants with silent mutations (see Figure 7D). The expression of sr-gfp-numb-full recovered the defects of Numb knockdown on integrin-b1 endocytosis (see Figure 7E), indicating that the effect of Numb RNAi is specific. The suppression of AP-2 or clathrin more profoundly inhibited integrin-b1, integrin-b3, and transferrin endocytosis under the same conditions (Figures 4A 4D). These results indicate that Numb functions in integrin endocytosis, but is not required for the process of clathrin-dependent endocytosis. Numb Is Required for Integrin-Stimulated Cell Migration We next investigated whether Numb controls integrindependent cell migration by using the Boyden chamber assay, which enabled us to count the cells migrating toward the extracellular matrix. HeLa cells moved toward fibronectin and collagen, but not toward nonphysiological matrix PDL, through the membrane in a growth-factorindependent manner (Figure 4E). The knockdown of Numb resulted in a marked reduction of integrin-stimulated cell migration toward fibronectin and collagen. More severe defects were observed in the AP-2- or clathrin-suppressed cells. The Numb-suppressed cells spread normally with a slight delay after reseeding (Figure 4F), suggesting that the suppression of Numb does not affect cell adhesion on the matrix and subsequent cell spreading. In contrast, suppression of AP-2 or clathrin significantly delayed the spreading. A time-course analysis, however, indicated that over 80% of the knockdown cells finally spread at 12 hr after reseeding (Figure S4C; data not shown). Thus, cell death could not account for the observed defects of integrin endocytosis and cell migration. In addition, the 18 Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc.
5 Figure 3. Numb Interacts with Integrin (A) Numb polarizes toward the wound edge and colocalizes with integrin-b1 in directionally migrating cells. Four hours after wounding, HeLa cells were incubated with anti-integrin-b1 antibody for 10 min before fixation. Arrows indicate colocalization of Numb and integrin-b1. The scale bar is 10 mm. (B) Numb interacts with integrin-b1 and integrin-b3 in vitro. GST-protein-immobilized beads were incubated with rat brain lysate. The GST fusion proteins are indicated by Coomassie brilliant blue (CBB) staining. Black dots indicate each intact band. (C) In vivo interaction of Numb with integrin-b1 in resting HeLa cells. Numb-suppressed cells, as well as AP-2- or clathrinsuppressed cells, slowly migrated into the wounding area (Figures 4G and 4H). Relatively weak defects in the wound-healing assay might be due to integrin-independent signals, such as growth factors in the serum and/or differences in the basic mechanism of migration, partially compensating for the defects of integrin endocytosis. These results indicate that Numb-mediated integrin endocytosis is not required for cell spreading, but is instead required for cell migration toward integrin substrates. Numb Binds to the PAR Polarity Complex, PAR-3 To further explore the regulatory mechanisms underlying the polarized localization and functions of Numb, we investigated the interaction of Numb with the PAR polarity complex, composed of PAR-3, PAR-6, and apkc, because the PAR polarity complex localizes at the leading edge and functions in polarized cell migration (Macara, 2004; Suzuki and Ohno, 2006). Numb coimmunoprecipitated PAR-3 and apkc in HeLa cells (Figure 5A) and in rat brain lysate (Figure S5A). Reciprocally, Numb was coimmunoprecipitated with PAR-3 and apkc, indicating that a part of Numb physiologically forms a complex with PAR-3 and apkc. GST-Numb-PTB, but not GST- Numb-PRR, efficiently precipitated myc-par-3-full and myc-apkcl-wt and weakly precipitated myc-par-6- WT (Figure 5B). To examine which region of PAR-3 is responsible for the interaction with Numb, various deletion fragments of PAR-3 were tested. Endogenous Numb specifically interacted with GST-PAR-3-4N and GST- PAR-3-4N/1, but not with GST-PAR-6-WT (Figure 5C). Recombinant GST-Numb-PTB bound to MBP-PAR-3-4N in vitro (Figure S5B), indicating that Numb directly binds to PAR-3. Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc. 19
6 Figure 4. Numb Is Required for Integrin Endocytosis and Integrin-Stimulated Cell Migration (A) Knockdown of Numb inhibits integrin endocytosis in directionally migrating cells. Four hours after wounding, HeLa cells were incubated with antiintegrin-b1 antibody for 20 min. Internalized integrin-b1 and F-actin are shown in red and blue, respectively. Images represent projection views of several confocal sections that covered the middle region of cells. The scale bar is 10 mm. (B) Quantitative analysis of the internalized integrin-b1 shown in (A). Total immunofluorescent intensity of internalized integrin-b1 was measured in the front cells toward the wounding area. (C) Suppression of Numb inhibits integrin endocytosis, but not transferrin endocytosis. HeLa cells were reseeded on PDL-coated coverslips for 1 hr, followed by an internalization assay. Internalized marker and F-actin are shown in red and blue, respectively. Images represent projection views of several confocal sections that covered the middle region of cells. The scale bar is 20 mm. (D) Quantitative analysis of the transferrin and integrin endocytosis shown in (C). Endocytosis-positive cells were scored compared with control cells as described in Experimental Procedures. Asterisks indicate a significant difference (p < 0.01) from the value of control siscramble-transfected cells. (E) Suppression of Numb impairs integrin-stimulated cell migration. HeLa cells were transfected with the indicated sirna, and the Boyden chamber migration assay toward poly-d-lysin (PDL), fibronectin (FN), or collagen (Col) was performed. Asterisks indicate a significant difference (p < 0.01) from the value of control siscramble-transfected cells. (F) Suppression of Numb does not affect cell spreading. HeLa cells were replated on PDL for 3 hr or fibronectin and collagen for 45 min. (G) Knockdown of Numb inhibits cell migration in a wound-healing assay. Images were captured shortly after wounding (upper panels) and 6 hr after wounding (lower panels). Arrowheads indicate the cell front. The scale bar is 30 mm. (H) Quantitative analysis of the wound-healing assay in (G). The migration distance over 6 hr was evaluated as described in Experimental Procedures. apkc Phosphorylates Numb In Vitro and In Vivo We next investigated whether Numb is phosphorylated by apkc in vitro. GST-Numb-PTB, GST-Numb-NT, and GST- Numb-PTB/N, all of which contain most of the N-terminal part of Numb, were phosphorylated by apkc in vitro (Figures 5D and 5E). The N-terminal part of Numb contains one putative PKC phosphorylation site (S-X-[R/K]) at Ser7, and Ala substitution of Ser7 abolished the phosphorylation of GST-Numb-PTB. The C-terminal part of Numb, GST-Numb-PRR and GST-Numb-PRR/1, was also phosphorylated by apkc. The Numb-PRR/1 region contains four putative PKC phosphorylation sites. We produced a series of point mutants and found that both Ser265 and Ser284 of Numb were involved in phosphorylation by apkc (Figure 5F). When any one or both of these sites were mutated to Ala, full-length Numb was still phosphorylated (Figure 5G). Mutations in all three sites (Numb-full-3A), however, abolished the phosphorylation by apkc, indicating that three Ser residues are the major sites of phosphorylation by apkc in vitro. These three Ser 20 Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc.
7 Figure 5. Numb Binds to PAR-3 and Is a Direct Target of apkc (A) Coimmunoprecipitation of Numb and the PAR complex from HeLa cell lysate. (B) Numb interacts with PAR-3 and apkc in vitro. GST-protein-immobilized beads were incubated with COS7 cell lysate expressing the indicated constructs. (C) Mapping of the PAR-3 region that is required for binding to Numb. (D) Deletion and point mutants of Numb for a phosphorylation assay with apkc. (E G) Numb is directly phosphorylated by apkc at three serine residues. Purified (E) GST-Numb deletion mutants, (F) GST-Numb-PRR point mutants, and (G) GST-Numb-full point mutants were incubated with recombinant apkcz in the presence of [ 32 P]ATP in vitro. The gels were stained with a silver staining to visualize GST fusion proteins (lower panels), followed by autoradiography (upper panels). Red dots indicate each intact band, and several bands below red dots are all degradation products. (H) Alignment of the three apkc phosphorylation sites in Numb homologs (human, chick, Drosophila, C. elegans) by ClustalW. Identical and similar amino acids are shown in violet and blue, respectively. residues are well conserved from human to chick, Drosophila, and Caenorhabditis elegans (Figure 5H), suggesting that phosphorylation may be conserved (Zhang et al., 2001; Smith et al., 2007). To prove in vivo phosphorylation of Numb, we generated the phospho-specific antibody against Ser7 (antips7-numb; Figures S5C and S5D). We detected the phosphorylation of endogenous Numb weakly in HeLa cells under normal growth conditions (Figure 6B). However, treatment with calyculin-a, a phosphatase inhibitor for PP1 and PP2A, increased the phosphorylation level of Numb in a time-dependent manner, and that increase was partially suppressed by the PKC inhibitor (Figure 6A). Thus, a certain population of Numb is constitutively phosphorylated and rapidly dephosphorylated by protein phosphatases, and the total phosphorylation level of Numb is regulated by both activities. To examine whether endogenous apkc is responsible for the phosphorylation of Numb in vivo, we suppressed the expression of both apkcl and apkcz by sirnas, because both apkc isoforms are supposed to phosphorylate Numb in vivo. Knockdown of apkc reduced, although not completely, the phosphorylation of Numb (Figure 6B), indicating that Numb is phosphorylated by apkc in vivo. The knockdown of PAR-3 partially reduced the phosphorylation, suggesting that PAR-3 functions as a scaffold that binds both apkc and Numb. apkc Regulates the Localization and Function of Numb We next examined whether phosphorylation of apkc affects the localization and functions of Numb. The expression of apkc-wt caused the disappearance of Numb puncta from the substratum-facing surface, Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc. 21
8 Figure 6. apkc Controls Subcellular Localization and Functions of Numb (A) Numb is phosphorylated and dephosphorylated in vivo. HeLa cells were treated with 0.1 mm calyculin-a with control DMSO or 10 mm PKC inhibitor (bisindolymaleimide [Bis]) for the indicated time periods. (B) Endogenous apkc phosphorylates Numb in vivo. HeLa cells were treated with 0.1 mm calyculin-a (Cal-A) for 5 min before lysis, where indicated. (C) Delocalization of Numb from CCSs by apkc. The arrow indicates an apkc-wt-expressing cell. The scale bar is 10 mm. (D) Localization of Numb to CCSs is dependent on its phosphorylation state. Experimental conditions were the same as those shown in Figure 1D. The scale bar is 5 mm. (E) Subcellular fractionation of GFP-Numb mutants. HeLa cell lysates (H) were fractionated into 0.5% Triton X-100 soluble (S) or insoluble (P) fractions. An equal volume of each lysate was analyzed by immunoblotting. RhoGDI was used as a soluble control. (F) Phosphorylated Numb reduces binding affinity to integrin-bs, PAR-3, and a-adaptin. GST proteins were incubated with recombinant apkcz in the presence or absence of ATP, and then mixed with rat brain lysate. (G) Mislocalization of Numb to both the apical and basal surface by apkc knockdown. HeLa cells transfected with siscramble or siapkc were replated on fibronectin-coated coverslips. (H) Numb is phosphorylated by apkc during migration. Confluent HeLa cells transfected with the indicated sirna were scratched for the woundhealing assay and were incubated for the indicated time periods. 0 hr indicates before wounding. (I) Phosphorylated Numb after wounding distributes in soluble fraction. (J) Knockdown of apkc impairs polarized distribution of Numb and a-adaptin. Four hours after wounding, HeLa cells transfected with siapkc were fixed and stained. The cell boundary (each cell morphology) was traced according to the staining of F-actin and a phase-contrast image (data not shown). The scale bars are 10 mm. (K) Quantitative analysis for the localization of Numb and a-adaptin in (J). Regions 1 5 are the same as those shown in the schematic in Figure 2B. whereas AP-2-positive CCSs were still observed (Figure 6C) (Smith et al., 2007). A phospho-mimic mutant form of Numb, in which all three apkc phosphorylation sites were mutated to aspartic acid (GFP-Numb-full-3D), consistently reduced the localization to CCSs even in the presence of the a-adaptin-binding DPF motif at the C terminus (Figure 6D; Figure S2B). Complementary biochemical fractionation confirmed that GFP-Numbfull-3D was mainly distributed in the soluble fraction, compared with GFP-Numb-full (Figure 6E). To elucidate the molecular mechanisms for the regulation of Numb by apkc, we incubated GST-Numb-full with recombinant apkcz in the presence or absence of ATP and then mixed with tissue lysate. Phosphorylated Numb significantly reduced the binding affinity to integrin-b1, integrin-b3, PAR-3, and a-adaptin (Figure 6F). GST-Numb-full-3D consistently decreased the binding to integrin-b1 and integrin-b3 (Figure S5E). Because the apkc phosphorylation sites of Numb do not overlap either with the PTB domain or the C-terminal AP-2-binding motif, 22 Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc.
9 we performed subdomain analysis to uncover the regulatory mechanism. However, we did not detect any binding between the N-terminal and C-terminal fragments we tested (the constructs used are shown in Figure 5D; data not shown). Thus, phosphorylation seems to affect a conformational change of Numb that sequesters the binding site of Numb to integrin and the AP-2 complex. The N- terminal part of Numb contains clustered acidic amino acids and binds to phospholipids (Dho et al., 1999; Zhang et al., 2001). The phosphorylation at Ser7 might reduce the binding affinity to phospholipids and thereby release Numb from the plasma membrane and receptors in vivo. Taken together, these results suggest that the functions of Numb are negatively regulated by apkc phosphorylation, and that Numb localization to CCSs is controlled by both AP-2 binding and its phosphorylation state. As expected, knockdown of apkc by sirnas or the expression of a dominant-negative mutant apkc-kinase dead (KD) did not impair the localization of Numb to CCSs (data not shown). After replating on integrin substrates, however, inactivation of apkc caused the mislocalization of Numb to CCSs at the apical cell surface in addition to those at the substratum-facing surface (Figure 6G). GFP-Numb-full-3A, a nonphosphorylated form of Numb, more efficiently localized to each CCS at both the apical and basal surface (Figure 6D; Figures S2B and S2D) and was distributed in an insoluble pellet fraction (Figure 6E). Thus, kinase activity of apkc seems to be required for the preferential localization of Numb to the CCSs at the substratum-facing surface. Integrin-mediated adhesions at the cell front activate Cdc42 and apkc in migrating cells (Etienne-Manneville and Hall, 2001). apkc plays a role in the establishment of cell polarization and directional cell migration by phosphorylation of several target proteins. We found that the phosphorylation level of Numb actually increased after wounding, and that this increase was partially suppressed by the knockdown of apkc and PAR-3 (Figure 6H; Figure S5F). Phosphorylated Numb after wounding was distributed in the soluble fraction (Figure 6I). Furthermore, the suppression of apkc impaired the polarized distribution of Numb and a-adaptin toward the leading edge after wounding (Figures 6J and 6K). It remains unclear whether knockdown of apkc directly affects Numb localization or indirectly affects it through actin filaments and integrin adhesions. Similar mislocalization was observed upon PAR-3 knockdown (data not shown). Functional Significance of Numb Phosphorylation in Integrin Endocytosis and Cell Migration Finally, we examined the functional significance of Numb phosphorylation by apkc. We transfected Numb and apkc mutants in HeLa cells and monitored integrin endocytosis and cell migration. GFP-Numb-PTB appeared to localize to the plasma membrane, but not to CCSs, and specifically inhibited integrin endocytosis and integrinstimulated migration (Figures 7A 7C). However, a phospho-mimic, Numb-PTB-S7D, diffusely localized in the cytoplasm and partially lost the inhibitory effects of Numb-PTB, suggesting that Numb-PTB, but not Numb- PTB-S7D, functions as a dominant-negative mutant interfering with Numb-integrin binding. In contrast, expression of Numb-PRR inhibited both integrin and transferrin endocytosis and integrin-stimulated migration (Figures 7B and 7C). These defects were largely abolished by mutations in the AP-2/Eps15-binding DPF/NPF motifs (Numb-PRR- DLA/NLA), indicating that Numb-PRR simply perturbs the AP-2 complex. If apkc negatively regulates Numb function in vivo, ectopic activation of apkc could inhibit integrin endocytosis. Indeed, expression of apkc-wt impaired integrin endocytosis, but not transferrin endocytosis (Figure 7B), whereas apkc-kd only slightly suppressed integrin endocytosis. Both apkc-wt and apkc-kd suppressed integrin-stimulated cell migration (Figure 7C), suggesting that proper activation of apkc at a defined area seems to be important for integrin-stimulated cell migration. The reason why apkc-kd more profoundly suppressed cell migration than apkc-wt could be due to the fact that apkc controls cell polarization and migration through several targets (Suzuki and Ohno, 2006). Knockdown of apkc and PAR-3 also suppressed integrin endocytosis and cell migration (Figure S6). Taken together, these results suggest that Numb phosphorylation by apkc is important for integrin endocytosis and integrin-stimulated cell migration. To examine the physiological significance of Numb phosphorylation, we performed rescue experiments with Numb mutants (Figure 7D). The expression of sr-gfp- Numb-PTB, GFP-Numb-PRR, and sr-gfp-numb-full- DLA/NLA did not rescue the defects (Figures 7E 7G), indicating that both the integrin-binding domain and the localization domain to CCSs are essential for the functions of Numb. sr-gfp-numb-full-3a did not rescue the defects, similar to sr-gfp-numb-full-3d, raising the possibility that a nonphosphorylated Numb mutant does not function as a constitutively active form. We monitored the behavior of GFP-Numb mutants over time. Puncta labeled by GFP-Numb-full displayed cycles of appearance and disappearance at the substratum-facing surface (Figure S7A), which resembles the phenomena reported for GFP-clathrin or GFP-a-adaptin (Perrais and Merrifield, 2005), suggesting that the appearance and disappearance of puncta would partially correlate with the formation of CCSs and the uncoating of clathrin-coated vesicles, respectively. However, many puncta of GFP-Numb-full-3A were static and persistent throughout the observation (Figure S7B). Expression of Numb-full-3A indeed inhibited both integrin and transferrin endocytosis as well as cell migration (Figures 7B and 7C). Taken together, these results suggest that cycling of phosphorylation and dephosphorylation of Numb is important for the functions of Numb in integrin endocytosis and directional cell migration. DISCUSSION Our observations indicate that Numb localizes at a part of CCSs and functions in integrin endocytosis as a cargo- Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc. 23
10 Figure 7. Functional Significance of Numb in Integrin Endocytosis and Cell Migration (A) Numb-PTB, but not Numb-PTB-S7D, inhibits integrin-b1 endocytosis. HeLa cells were reseeded on PDL-coated coverslips for 1 hr, followed by an internalization assay with anti-integrin-b1 antibody (bottom). Arrows indicate transfected cells. The scale bar is 20 mm. (B) Effects of apkc and Numb mutants on integrin and transferrin endocytosis. Under the same conditions in (A), endocytosis-positive cells with perinuclear accumulation of internalized marker were counted. Asterisks and double asterisks indicate a significant difference (p < 0.05 and p < 0.01, respectively) from the value of control GFP-transfected cells. D/N, DLA/NLA mutant. (C) Effects of apkc and Numb mutants on integrin-stimulated cell migration. The Boyden chamber migration assay toward fibronectin was performed. (D) Expression of the sirna-resistant (sr)-numb construct in HeLa cells. (E) Quantitative analysis for the effects of sr-numb mutants on integrin-b1 endocytosis. Asterisks indicate a significant difference (p < 0.01) from the value of control siscramble with GFP-transfected cells. (F) Effects of sr-numb mutants in integrin-stimulated cell migration. HeLa cells were transfected with the indicated sirna and GFP constructs along with GST, and the Boyden chamber migration assay toward fibronectin was performed. Membranes were stained with anti-gst antibody to visualize migrated cells. The scale bar is 100 mm. (G) Quantitative analysis for the migration assay shown in (F). Asterisks indicate a significant difference (p < 0.01) from the value of control siscramble with GFP-transfected cells. selective adaptor. Integrin is thought to be recycled from the tail to the front of migrating cells by endocytosis. However, many focal adhesions or focal complexes formed at the cell front disassemble behind the F-actin-rich lamellipodia (Webb et al., 2002; Ridley et al., 2003). Numb mainly accumulated behind lamellipodia, although a certain population of Numb still remained and colocalized with integrin at the trailing edge. In addition, localization of Numb among CCSs correlated with the position of integrin adhesions, supporting the role of Numb in integrin endocytosis. Talin is a key molecule that tethers integrin to components of focal adhesions and actin stress fibers and is critical for focal-adhesion disassembly (Franco et al., 2004). Mutation of a conserved tyrosine residue within the integrinb3 intracellular domain abolished the binding of both talin and Numb (Calderwood et al., 2003), suggesting that talin and Numb cannot bind to integrin simultaneously. Consistent with these observations, we were unable to detect the interaction of Numb with talin. In addition, the binding of Numb to integrin does not activate the integrin extracellular domain, whereas the binding of talin does activate it (Calderwood et al., 2002). The overexpression or knockdown of Numb did not directly affect cell adhesion. Thus, it appears that Numb does not actively promote focal-adhesion disassembly, but rather recruits free integrins without the components of focal adhesions to the 24 Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc.
11 AP-2 complex for internalization. Preferential localization of Numb around focal adhesions at the substratum-facing surface would facilitate recruitment of integrin during focal adhesion disassembly. Recent genetic screening isolated Numb as a mutant defective for peripheral glia migration along axons in Drosophila (Edenfeld et al., 2007). Migration defects of postmitotic neurons have been described in Numbknockout mice (Klein et al., 2004), indicating that Numb regulates particular cell migration in vivo. However, the defects of Numb knockdown on integrin endocytosis and cell migration are less marked than those of AP-2 and clathrin knockdown, suggesting that another adaptor molecule(s) may function in integrin endocytosis. A good candidate is disabled-2 (Dab2), which has a similar domain structure as Numb and binds to both components of clathrin-mediated endocytosis and to integrin-b (Morris and Cooper, 2001; Mishra et al., 2002; Calderwood et al., 2003). Dab2 is expressed in HeLa cells and positively controls cell adhesion and spreading (Rosenbauer et al., 2002). In contrast to Numb, Dab2 appears to preferentially localize to the apical surface (Morris and Cooper, 2001). Thus, Numb and Dab2 may coordinately function in integrin endocytosis in different subcellular compartments for cell motility. How does Numb localize at the substratum-facing surface and polarize toward the leading edge? Integrin adhesions could activate several intracellular signaling events and promote protein transport to adhesion sites by targeting microtubules and linking actin stress fibers (Ridley et al., 2003; Small and Kaverina, 2003). We found that the actin cytoskeleton and/or adhesion itself are important for the preferential localization of Numb around adhesions. However, Numb still localized at the substratum-facing surface in the presence of cytochalasin-d, indicating that an additional mechanism may exist. Our observations indicate that direct phosphorylation by apkc may be a part of the regulatory mechanism underlying Numb localization at the substratum-facing surface. In addition, polarized localization of Numb toward the leading edge was lost upon apkc knockdown. In support of these observations, asymmetric localization of Numb in Drosophila has been shown to be dependent on cortical actomyosin and the polarized localization/function of apkc and PAR-3 (Lu et al., 1999; Betschinger and Knoblich, 2004; Roegiers and Jan, 2004). Conclusive evidence will require isolation of the responsible motor(s) and anchor protein(s) for specific Numb localization. We found that Numb-full-3A did not function as a constitutively active form that promotes integrin endocytosis and cell migration, but rather inhibited these processes. Similarly, both the phospho-mimic and nonphosphorylated form of m2-adaptin, which is phosphorylated by AAK1, inhibit transferrin endocytosis (Conner and Schmid, 2002), suggesting that clathrin-mediated endocytosis is tightly controlled by cycles of phosphorylation and dephosphorylation. Additional phosphorylation during endocytosis may be required for the dissociation of Numb from the binding proteins, integrin-b, and a-adaptin. Numb is indeed phosphorylated by several PKCs and CaMKs (Tokumitsu et al., 2005; Smith et al., 2007), and phosphatase inhibitor dramatically increases the phosphorylation level of Numb. Thus, local phosphorylation and dephosphorylation seems to allow Numb to localize defined CCSs around adhesion sites. Trafficking of internalized integrin is regulated by growth factors and the extracellular matrix through several adaptors/kinases, including PI3-kinase, PKB/Akt, GSK3b, and PKCs (Jones et al., 2006; Pellinen and Ivaska, 2006). Several growth factors and adhesions indeed promote the recycling of integrins, leading to the upregulation of cell-surface expression (Powelka et al., 2004; Woods et al., 2004; Li et al., 2005), whereas treatment of cells with PDGF does not affect the internalization rate of integrin (Roberts et al., 2001). The degree of colocalization and interaction of Numb with integrin-b1 was not significantly altered in HeLa cells before and after wounding (data not shown). These data suggest that Numb functions constitutively in integrin endocytosis, although it is possibile that polarized migration promotes the internalization rate and amount of integrin endocytosis. It might be difficult to detect the changes in the interaction of Numb and integrin during migration due to the nature of rapid cycling of endocytosis and exocytosis and possibly due to the transient interaction. It has been reported that the inhibition of directional membrane trafficking causes membrane extension in all directions (Shirane and Nakayama, 2006). Membrane trafficking controls the directionality of migrating cells (Prigozhina and Waterman-Storer, 2006). Taking into account the fact that Numb localization becomes polarized coincidently with directional migration, the subcellular region at which integrin is internalized and the subsequent coupling with the recycling processes could be important for efficient cell migration suitable for the particular environment. EXPERIMENTAL PROCEDURES Materials and Biochemical Experiments See Supplemental Data. Cell Culture and Immunofluorescence Analysis ECV304, HeLa, and Vero cells were cultured in DMEM with 10% fetal bovine serum, and transfection was performed by using Lipofectamin or Lipofectamin2000 (Invitrogen), according to the manufacturer s instructions. We used HeLa cells for the functional assay, because transfection and knockdown efficiency were less effective in Vero and ECV304 cells. Cells were fixed at indicated periods with 3.7% formaldehyde in PBS for 10 min at room temperature, followed by treatment for 10 min with 0.2% Triton X-100 that contained 2 mg/ml BSA or cold methanol. The cells were observed with a confocal laser microscope (LSM510; Carl Zeiss) built around an Axiovert 100 M (Carl Zeiss) to acquire images for presentation and quantification or with a Zeiss Axiophoto microscope (Carl Zeiss) for counting cell morphology and internalized markers. All presented images represent a single confocal section (thickness of mm) corresponding to an area of the plasma membrane close to the substrate (substratumfacing surface), except as noted in the figure legend. The x-z images are projection views of confocal sections (thickness of mm, width of 3 5 mm). Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc. 25
12 sirna Transfection Oligonucleotide sirna duplex was synthesized by Japan Bio Service (Saitama, Japan). sirna sequences were as follows: siscramble, 5 0 -CAGTCGCGTTTGCGACTGG-3 0 ; sinumb, 5 0 -GGACCTCATAGTT GACCAG-3 0 ; siapkcz, 5 0 -AGAGGATCGACCAGTCAGA-3 0 ; siapkcl, 5 0 -CGAACAGCTCTTCACCATG-3 0 ; and sipar-3, 5 0 -GGCATGGA GACCTTGGAAG-3 0. sirna sequences of m2-adaptin and clathrin-heavy-chain were used for targeting AP-2 complex or clathrin, as described by Motley et al. (2003). sr Numb was generated by changing the targeted sequences of sinumb to the sequence 5 0 -GGACCTCATTGTAGACCAG-3 0 (mutated nucleotides are underlined) by PCR-based site-directed mutagenesis. The transfection of sirna in HeLa cells was carried out with Oligofectamine reagent (Invitrogen), according to the manufacturer s instructions. Cells were analyzed at 3 days after transfection. For rescue experiments, cells were transfected with the intended plasmid, along with sirna at 36 hr after transfection, by using Lipofectamin (Invitrogen). At 24 hr after transfection, cells were fixed or analyzed as follows. Quantification of Protein Localization The images of cells were captured with LSM 510, and fluorescence intensity quantification was performed with ImagePro analysis (Media Cybernetics). For the distribution of proteins in migrating cells, fluorescent intensity was measured in five equally divided regions as a function of the direction of the migration in each cell. Average fluorescence intensity per area was determined by dividing the sum of all pixel intensities by the measured area after subtraction of the background. Data were compared as a function of the percentage of total intensity in the five measured regions. All compared images were acquired under identical parameters. Data represent the mean and SEM of cells. For the localization of proteins at the adhesion area, we adapted a method described by Ezratty et al. (2005). Total fluorescent intensity was measured in an adhesion area (5 mm circle in a diameter containing a visible adhesion marker, either vinculin or GFP-zyxin) and an adjacent nonadhesion area (same circle without visible adhesion marker), and the background signals were subtracted. All compared images were acquired under identical parameters. Data represent the mean and SEM of a total areas from cells in each condition. Cell Motility Assay Integrin-stimulated cell migration was evaluated by the modified Boyden chamber migration assay by using Transwell (Costar) 24-well tissue culture plates composed of a polycarbonate membrane with 5 mm pores. The lower side of the membrane was coated with 10 mg/ml PDL (Sigma), 10 mg/ml fibronectin (BD Biosciences), or 40 mg/ml collagen type I (Sigma) for 4 hr and was blocked with serum-free DMEM containing 0.5% bovine serum albumin (BSA) for 1 hr at 37 C. HeLa cells were suspended in 0.25% trypsin, neutralized with 10 mg/ml soybean trypsin inhibitor (Sigma), and harvested in DMEM containing 0.5% BSA. The cells were seeded on the upper chamber at cells and were incubated for 4 hr. The cells that migrated to the lower side of the membrane were fixed and stained with phalloidin or anti-gst antibody against cotransfected GST as a fill marker for rescue experiments. Migrating cells were evaluated by the average fluorescence intensity of phalloidin or GST signals in six randomly chosen fields for each condition. All compared images were acquired under identical parameters. The data are normalized with the average fluorescence intensity of GST signals in each aliquot plated on PDLcoated coverslips. For the spreading assay, cells were replated on collagen-, fibronectin-, or PDL-coated coverslips, as described above, in serum-free DMEM containing 0.5% BSA for the indicated time period, fixed, and processed for immunofluorescence analysis. At least 100 cells in each condition were counted at each time point. Data represent the mean and SD of at least three independent experiments. For the wound-healing assay, cells were replated on dishes or collagen-coated coverslips at a high density. At 24 hr after seeding, cells were scratched by pipette tip or 18G-needle and incubated for the indicated time period in the presence of serum. Phase-contrast images corresponding to the same area in a glass-bottom dish were acquired shortly after and 6 hr after wounding and were superimposed. The average migration distance was determined by dividing the migrating area evaluated by tracing the cell front at each time point by the measured width. Data represent the mean and SD of at least 30 independent fields in each condition from 2 independent experiments. For biochemical analysis, 50% of the cells were scraped away by wounding several times across the dish, and the remaining attached cells were assessed. Internalization Assay For the analysis of integrin internalization, we adapted an immunohistochemical assay based on those described previously (Kamiguchi and Lemmon, 2000; Roberts et al., 2001; Nishimura et al., 2003). HeLa cells were reseeded on PDL-coated coverslips in DMEM containing 0.5% BSA. At 1 hr after reseeding, live cells were incubated with mouse anti-integrin-b1 (MAB1981, Chemicon) or rabbit anti-integrin-b3 antibodies (AB1932, Chemicon), which recognize the extracellular domain of integrin, for 20 min at 37 C to allow for internalization of antibody-bound integrin. After being rinsed at 4 C, the cells were fixed and incubated with an excess amount of unconjugated anti-mouse or anti-rabbit IgG (Jackson Laboratories) to block the antibody remaining on the cell surface. The cells were permeabilized with 0.2% Triton X-100. Internalized integrins were then visualized with fluorescenceconjugated secondary antibody. To visualize cell-surface integrin, fixed cells were incubated with fluorescence-conjugated secondary antibody without permeabilization. For internalization of transferrin, cells were incubated with 20 mg/ml tetrametylrhodamine-conjugated transferrin (Molecular Probes) in culture medium for 10 min at 37 C. For quantification of the internalization assay, the total fluorescent intensity per cell area was measured in the front cells at the wound edge, according to the staining of F-actin. Data represent the mean and SEM of cells in each condition. All images were acquired under identical parameters. Data are representative of two independent experiments. For Figure 4D, the cell area was traced according to the staining of phalloidin or GST signals, and the mean fluorescence intensity per pixel of internalized marker was determined by dividing the sum of all pixel intensities by the cell area for at least 100 cells in each condition. Approximately 80% of the cells showed perinuclear accumulation of integrin or transferrin signals in siscramble-transfected cells or untreated cells. Thus, endocytosis-positive cells were scored as the percentage of cells that had a higher intensity than the lowest 20% of the control cells. All compared images were acquired under identical parameters. For Figure 7B, internalization-positive cells were counted as a cell with perinuclear accumulation of each internalized marker. Data represent the mean and SD of three independent experiments. Of note, our assay for integrin endocytosis cannot discriminate the place at which integrin is internalized throughout the plasma membrane, such as the apical and basal surfaces. In addition, it is possible that antibody treatment may have affected the internalization rate. Supplemental Data Supplemental Data include Supplemental Experimental Procedures, Supplemental References, and seven figures and are available at ACKNOWLEDGMENTS We thank S. Ohno, H. Saya, A. Tokunaga, and H. Okano for kindly providing materials; M. Amano, T. Yamaguchi, K. Kato, and N. Nishimura for technical help and preparing some materials; and T. Ishii for secretarial assistance. We are grateful to J.A. Knoblich for the use of his laboratory s facility and comments on the paper. This research 26 Developmental Cell 13, 15 28, July 2007 ª2007 Elsevier Inc.
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
Flag-Rac Vector V12 V12 N17 C40. Vector C40 pakt (T308) Akt1. Myc-DN-PAK1 (N-SP)
 a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
a b FlagRac FlagRac V2 V2 N7 C4 V2 V2 N7 C4 p (T38) p (S99, S24) p Flag (Rac) NIH 3T3 COS c +Serum p (T38) MycDN (NSP) Mycp27 3 6 2 3 6 2 3 6 2 min p Myc ( or p27) Figure S (a) Effects of Rac mutants on
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Stargazin regulates AMPA receptor trafficking through adaptor protein. complexes during long term depression
 Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Hong et al.,
 Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Journal of Cell Science Supplementary Material
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Figure S2. Verification of ihog and boi
 Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
Supplementary Figure S1. N-terminal fragments of LRRK1 bind to Grb2.
 Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Nature Structural & Molecular Biology: doi: /nsmb.1583
 Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Acetylation by GCN5 regulates CDC6 phosphorylation in the S-phase of the cell cycle Roberta Paolinelli 1,2, Ramiro Mendoza-Maldonado 2, Anna Cereseto 1 and Mauro Giacca 2 1 Molecular Biology Laboratory,
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or
 Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Figure 1: TDP-43 is subject to lysine acetylation within the RNA-binding domain a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table.
 Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit
 Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
Supplemental Information. Pacer Mediates the Function of Class III PI3K. and HOPS Complexes in Autophagosome. Maturation by Engaging Stx17
 Molecular Cell, Volume 65 Supplemental Information Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17 Xiawei Cheng, Xiuling Ma, Xianming Ding,
Molecular Cell, Volume 65 Supplemental Information Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17 Xiawei Cheng, Xiuling Ma, Xianming Ding,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
Supplementary Figure 1. The Hsp70 acetylation level is related to the co-chaperone binding of Hsp70 under various stress conditions.
 Supplementary Figure 1. The Hsp70 acetylation level is related to the co-chaperone binding of Hsp70 under various stress conditions. 1 (a) Etoposide treatment gradually changes acetylation level and co-chaperone
Supplementary Figure 1. The Hsp70 acetylation level is related to the co-chaperone binding of Hsp70 under various stress conditions. 1 (a) Etoposide treatment gradually changes acetylation level and co-chaperone
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG
 Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Supplementary Figure 1 Phosphorylated tau accumulates in Nrf2 (-/-) mice. Hippocampal tissues obtained from Nrf2 (-/-) (10 months old, 4 male; 2
 Supplementary Figure 1 Phosphorylated tau accumulates in Nrf2 (-/-) mice. Hippocampal tissues obtained from Nrf2 (-/-) (10 months old, 4 male; 2 female) or wild-type (5 months old, 1 male; 11 months old,
Supplementary Figure 1 Phosphorylated tau accumulates in Nrf2 (-/-) mice. Hippocampal tissues obtained from Nrf2 (-/-) (10 months old, 4 male; 2 female) or wild-type (5 months old, 1 male; 11 months old,
The role of the ninth and tenth type III domains of human fibronectin in cell adhesion
 ELSEVIER FEBS Letters 340 (1994) 197-201 FEBS 13711 LETTERS The role of the ninth and tenth type III domains of human fibronectin in cell adhesion Helen J. Mardon*, Kate E. Grant Nuffield Department of
ELSEVIER FEBS Letters 340 (1994) 197-201 FEBS 13711 LETTERS The role of the ninth and tenth type III domains of human fibronectin in cell adhesion Helen J. Mardon*, Kate E. Grant Nuffield Department of
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured
 Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
Supplementary Material
 Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
SUPPLEMENTARY INFORMATION
 The Supplementary Information (SI) Methods Cell culture and transfections H1299, U2OS, 293, HeLa cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. H1299 and 293 cells were
The Supplementary Information (SI) Methods Cell culture and transfections H1299, U2OS, 293, HeLa cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. H1299 and 293 cells were
Supplementary Fig.1 Luton
 Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
Supplementary Fig.1 Luton a 175 Brain Thymus Spleen Small Intestine Kidney Testis HeLa b 250 Lung Kidney MDCK c EFA6B si Control si Mismatch #637 #1564 #1770 83 62 47.5 175 IB: anti-efa6b #B1 130 66 Lysates
Figure S1 is related to Figure 1B, showing more details of outer segment of
 Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb
 Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Methanol fixation allows better visualization of Kal7. To compare methods for
 Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
SUPPLEMENTAL MATERIAL. Supplemental Methods:
 SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
Supplementary Figure 1. Drawing of spinal cord open-book preparations and DiI tracing. Nature Neuroscience: doi: /nn.3893
 Supplementary Figure 1 Drawing of spinal cord open-book preparations and DiI tracing. Supplementary Figure 2 In ovo electroporation of dominant-negative PlexinA1 in commissural neurons induces midline
Supplementary Figure 1 Drawing of spinal cord open-book preparations and DiI tracing. Supplementary Figure 2 In ovo electroporation of dominant-negative PlexinA1 in commissural neurons induces midline
Supplementary Information
 Supplementary Information promotes cancer cell invasion and proliferation by receptor-mediated endocytosis-dependent and -independent mechanisms, respectively Kensaku Shojima, Akira Sato, Hideaki Hanaki,
Supplementary Information promotes cancer cell invasion and proliferation by receptor-mediated endocytosis-dependent and -independent mechanisms, respectively Kensaku Shojima, Akira Sato, Hideaki Hanaki,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
supplementary information
 DOI: 10.1038/ncb1919 Mori et al. Supplementary Figure 1a-b a Characterization of the mdrg cells Anti-NF150 DIC (89%: N=154) 100µm Anti-S100 DIC 100µm b (9.1%: N=144) Characterization of the cortical neurons
DOI: 10.1038/ncb1919 Mori et al. Supplementary Figure 1a-b a Characterization of the mdrg cells Anti-NF150 DIC (89%: N=154) 100µm Anti-S100 DIC 100µm b (9.1%: N=144) Characterization of the cortical neurons
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression
 Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
Supplemental figures Supplemental Figure. 1. Silencing expression of Celsr3 by shrna. To examine the silencing effects of Celsr3 shrna, we co transfected 293T cells with expression plasmids for the shrna
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Paul et al.,
 Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Journal of Cell Science Supplementary Material
 Figure S4 A His-(RBCPS)T15 His-(_BCPS) T15 His-PXN His-PXN+T15 His-PXN+ (RB_PS)T15 Myc-Ub IP:Ni-NTA 6M GuHCL WB: anti-myc WB: anti-his (Ub)n His-PXN His-(RBCPS)T15 His-(_BCPS)T15 + + + + + + + + + -100
Figure S4 A His-(RBCPS)T15 His-(_BCPS) T15 His-PXN His-PXN+T15 His-PXN+ (RB_PS)T15 Myc-Ub IP:Ni-NTA 6M GuHCL WB: anti-myc WB: anti-his (Ub)n His-PXN His-(RBCPS)T15 His-(_BCPS)T15 + + + + + + + + + -100
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
14_integrins_EGFR
 α1 Integrin α1 -/- fibroblasts from integrin α1 knockout animals Figure S1. Serum-starved fibroblasts from α -/- 1 and +/+ mice were stimulated with 10% FBS for 30 min. (FBS) or plated on collagen I (CI)
α1 Integrin α1 -/- fibroblasts from integrin α1 knockout animals Figure S1. Serum-starved fibroblasts from α -/- 1 and +/+ mice were stimulated with 10% FBS for 30 min. (FBS) or plated on collagen I (CI)
MCF10A cells were trypsinized and attached onto fibronectin-coated petri dishes
 Supplemental Information Supplemental figure legends Figure S. Supplemental figure for Fig... Different methods of cell detachment similarly induce YP phosphorylation. MCF0 cells were trypsinized and attached
Supplemental Information Supplemental figure legends Figure S. Supplemental figure for Fig... Different methods of cell detachment similarly induce YP phosphorylation. MCF0 cells were trypsinized and attached
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
A RRM1 H2AX DAPI. RRM1 H2AX DAPI Merge. Cont. sirna RRM1
 A H2AX DAPI H2AX DAPI Merge Cont sirna Figure S1: Accumulation of RRM1 at DNA damage sites (A) HeLa cells were subjected to in situ detergent extraction without IR irradiation, and immunostained with the
A H2AX DAPI H2AX DAPI Merge Cont sirna Figure S1: Accumulation of RRM1 at DNA damage sites (A) HeLa cells were subjected to in situ detergent extraction without IR irradiation, and immunostained with the
J. Cell Sci. 128: doi: /jcs : Supplementary Material. Supplemental Figures. Journal of Cell Science Supplementary Material
 Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Han et al., http://www.jcb.org/cgi/content/full/jcb.201311007/dc1 Figure S1. SIVA1 interacts with PCNA. (A) HEK293T cells were transiently
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Han et al., http://www.jcb.org/cgi/content/full/jcb.201311007/dc1 Figure S1. SIVA1 interacts with PCNA. (A) HEK293T cells were transiently
supplementary information
 DOI: 10.1038/ncb2116 Figure S1 CDK phosphorylation of EZH2 in cells. (a) Comparison of candidate CDK phosphorylation sites on EZH2 with known CDK substrates by multiple sequence alignments. (b) CDK1 and
DOI: 10.1038/ncb2116 Figure S1 CDK phosphorylation of EZH2 in cells. (a) Comparison of candidate CDK phosphorylation sites on EZH2 with known CDK substrates by multiple sequence alignments. (b) CDK1 and
Supplemental Figure 1 Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical
 Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
by Neurobasal medium (supplemented with B27, 0.5mM glutamine, and 100 U/mL
 Supplementary Materials and methods Neuronal cultures and transfection The hippocampus was dissected from E8 rat embryos, dissociated, and neurons plated onto glass coverslips coated with poly-ornithine
Supplementary Materials and methods Neuronal cultures and transfection The hippocampus was dissected from E8 rat embryos, dissociated, and neurons plated onto glass coverslips coated with poly-ornithine
Supplementary data. sienigma. F-Enigma F-EnigmaSM. a-p53
 Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Supplementary data Supplemental Figure 1 A sienigma #2 sienigma sicontrol a-enigma - + ++ - - - - - - + ++ - - - - - - ++ B sienigma F-Enigma F-EnigmaSM a-flag HLK3 cells - - - + ++ + ++ - + - + + - -
Supplementary Figure 1. α-synuclein is truncated in PD and LBD brains. Nature Structural & Molecular Biology: doi: /nsmb.
 Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplementary Figure Legends
 Supplementary Figure Legends Supplementary Fig. 1. The third PDZ domain of PAR-3 and the C-terminal PDZ domain binding motif of VE-cadherin mediate the recruitment of PAR-3 to cell-cell contacts in cells.
Supplementary Figure Legends Supplementary Fig. 1. The third PDZ domain of PAR-3 and the C-terminal PDZ domain binding motif of VE-cadherin mediate the recruitment of PAR-3 to cell-cell contacts in cells.
Figure S1. USP-46 is expressed in several tissues including the nervous system
 Supplemental Figure legends Figure S1. USP-46 is expressed in several tissues including the nervous system Transgenic animals expressing a transcriptional reporter (P::GFP) were imaged using epifluorescence
Supplemental Figure legends Figure S1. USP-46 is expressed in several tissues including the nervous system Transgenic animals expressing a transcriptional reporter (P::GFP) were imaged using epifluorescence
Input. Pulldown GST. GST-Ahi1
 A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of
 Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
Supplementary Information Cytotoxicity of Botulinum Neurotoxins Reveals a Direct Role of Syntaxin 1 and SNAP-25 in Neuron Survival Lisheng Peng, Huisheng Liu, Hongyu Ruan, William H. Tepp, William H. Stoothoff,
GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts
 Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various
 Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
Supplementary Materials and Methods
 Supplementary Materials and Methods sirna sequences used in this study The sequences of Stealth Select RNAi for ALK and FLOT-1 were as follows: ALK sense no.1 (ALK): 5 -AAUACUGACAGCCACAGGCAAUGUC-3 ; ALK
Supplementary Materials and Methods sirna sequences used in this study The sequences of Stealth Select RNAi for ALK and FLOT-1 were as follows: ALK sense no.1 (ALK): 5 -AAUACUGACAGCCACAGGCAAUGUC-3 ; ALK
Recruitment of Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells
 SUPPLEMENTARY FIGURES Recruitment of Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells Niklas Engels, Lars Morten König, Christina Heemann, Johannes
SUPPLEMENTARY FIGURES Recruitment of Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells Niklas Engels, Lars Morten König, Christina Heemann, Johannes
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Moutin et al., http://www.jcb.org/cgi/content/full/jcb.201110101/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Tagged Homer1a and Homer are functional and display different
Supplemental material Moutin et al., http://www.jcb.org/cgi/content/full/jcb.201110101/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Tagged Homer1a and Homer are functional and display different
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Supplementary Information for. Regulation of Rev1 by the Fanconi Anemia Core Complex
 Supplementary Information for Regulation of Rev1 by the Fanconi Anemia Core Complex Hyungjin Kim, Kailin Yang, Donniphat Dejsuphong, Alan D. D Andrea* *Corresponding Author: Alan D. D Andrea, M.D. Alan_dandrea@dfci.harvard.edu
Supplementary Information for Regulation of Rev1 by the Fanconi Anemia Core Complex Hyungjin Kim, Kailin Yang, Donniphat Dejsuphong, Alan D. D Andrea* *Corresponding Author: Alan D. D Andrea, M.D. Alan_dandrea@dfci.harvard.edu
(phosphatase tensin) domain is shown in dark gray, the FH1 domain in black, and the
 Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Quantitative analysis of Bidirectional Signaling (qbids)
 Quantitative analysis of Bidirectional Signaling (qbids) EphB2 + cells were labeled independently with light (C 12 N 14 ) arginine and lysine or heavy (C 13 N 15 ) arginine and lysine ephrin-b1 + cells
Quantitative analysis of Bidirectional Signaling (qbids) EphB2 + cells were labeled independently with light (C 12 N 14 ) arginine and lysine or heavy (C 13 N 15 ) arginine and lysine ephrin-b1 + cells
Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated
 Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
Supplementary Figure Legends Supplementary Fig. 1. Multiple five micron sections of liver tissues of rats treated with either vehicle (left; n=3) or CCl 4 (right; n=3) were co-immunostained for NRP-1 (green)
Supplementary Materials
 Supplementary Materials Supplementary Figure 1. PKM2 interacts with MLC2 in cytokinesis. a, U87, U87/EGFRvIII, and HeLa cells in cytokinesis were immunostained with DAPI and an anti-pkm2 antibody. Thirty
Supplementary Materials Supplementary Figure 1. PKM2 interacts with MLC2 in cytokinesis. a, U87, U87/EGFRvIII, and HeLa cells in cytokinesis were immunostained with DAPI and an anti-pkm2 antibody. Thirty
PHT1;2-CFP YFP-PHF + PHT1;2-CFP YFP-PHF
 YFP-PHF1 CFP-PHT1;2 PHT1;2-CFP YFP-PHF + PHT1;2-CFP YFP-PHF + CFP-PHT1;2 Negative control!-gfp Supplemental Figure 1: PHT1;2 accumulation is PHF1 dependent. Immunoblot analysis on total protein extract
YFP-PHF1 CFP-PHT1;2 PHT1;2-CFP YFP-PHF + PHT1;2-CFP YFP-PHF + CFP-PHT1;2 Negative control!-gfp Supplemental Figure 1: PHT1;2 accumulation is PHF1 dependent. Immunoblot analysis on total protein extract
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/404/ra120/dc1 Supplementary Materials for The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1 Akihiro
www.sciencesignaling.org/cgi/content/full/8/404/ra120/dc1 Supplementary Materials for The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1 Akihiro
Supplementary Information
 Supplementary Information Supplementary Figure 1: Over-expression of CD300f in NIH3T3 cells enhances their capacity to phagocytize AC. (a) NIH3T3 cells were stably transduced by EV, CD300f WT or CD300f
Supplementary Information Supplementary Figure 1: Over-expression of CD300f in NIH3T3 cells enhances their capacity to phagocytize AC. (a) NIH3T3 cells were stably transduced by EV, CD300f WT or CD300f
T H E J O U R N A L O F C E L L B I O L O G Y
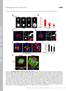 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Supplementary Figure 1. Expressions of stem cell markers decreased in TRCs on 2D plastic. TRCs were cultured on plastic for 1, 3, 5, or 7 days,
 Supplementary Figure 1. Expressions of stem cell markers decreased in TRCs on 2D plastic. TRCs were cultured on plastic for 1, 3, 5, or 7 days, respectively, and their mrnas were quantified by real time
Supplementary Figure 1. Expressions of stem cell markers decreased in TRCs on 2D plastic. TRCs were cultured on plastic for 1, 3, 5, or 7 days, respectively, and their mrnas were quantified by real time
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.
 Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
Supplementary Information
 Supplementary Information Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction Hideto Takahashi 1, Kei-ichi Katayama 2, Kazuhiro Sohya 3,4, Hiroyuki Miyamoto 4,5,
Supplementary Information Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction Hideto Takahashi 1, Kei-ichi Katayama 2, Kazuhiro Sohya 3,4, Hiroyuki Miyamoto 4,5,
ASPP1 Fw GGTTGGGAATCCACGTGTTG ASPP1 Rv GCCATATCTTGGAGCTCTGAGAG
 Supplemental Materials and Methods Plasmids: the following plasmids were used in the supplementary data: pwzl-myc- Lats2 (Aylon et al, 2006), pretrosuper-vector and pretrosuper-shp53 (generous gift of
Supplemental Materials and Methods Plasmids: the following plasmids were used in the supplementary data: pwzl-myc- Lats2 (Aylon et al, 2006), pretrosuper-vector and pretrosuper-shp53 (generous gift of
Promotion of HDF Cell Attachment and Proliferation
 Promotion of HDF Cell Attachment and Proliferation Objectives To qualitatively assess the effect of fibronectin (Fn) on HDF cell attachment Fn Attachment Assay To observe HDF cell proliferation and position
Promotion of HDF Cell Attachment and Proliferation Objectives To qualitatively assess the effect of fibronectin (Fn) on HDF cell attachment Fn Attachment Assay To observe HDF cell proliferation and position
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity
 Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
Supplemental figures (1-9) Gu et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity Supplemental figure 1. The quantification method to determine the
Xu et al., Supplementary Figures 1-7
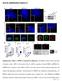 Xu et al., Supplementary Figures 1-7 Supplementary Figure 1. PIPKI is required for ciliogenesis. (a) PIPKI localizes at the basal body of primary cilium. RPE-1 cells treated with two sirnas targeting to
Xu et al., Supplementary Figures 1-7 Supplementary Figure 1. PIPKI is required for ciliogenesis. (a) PIPKI localizes at the basal body of primary cilium. RPE-1 cells treated with two sirnas targeting to
HPV E6 oncoprotein targets histone methyltransferases for modulating specific. Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu,
 1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
1 HPV E oncoprotein targets histone methyltransferases for modulating specific gene transcription 3 5 Chih-Hung Hsu, Kai-Lin Peng, Hua-Ci Jhang, Chia-Hui Lin, Shwu-Yuan Wu, Cheng-Ming Chiang, Sheng-Chung
Supplemental Material
 Supplemental Material 1 Figure S1. Phylogenetic analysis of Cep72 and Lrrc36, comparative localization of Cep72 and Lrrc36 and Cep72 antibody characterization (A) Phylogenetic alignment of Cep72 and Lrrc36
Supplemental Material 1 Figure S1. Phylogenetic analysis of Cep72 and Lrrc36, comparative localization of Cep72 and Lrrc36 and Cep72 antibody characterization (A) Phylogenetic alignment of Cep72 and Lrrc36
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
Supplemental Materials and Methods
 Supplemental Materials and Methods Co-immunoprecipitation (Co-IP) assay Cells were lysed with NETN buffer (20 mm Tris-HCl, ph 8.0, 0 mm NaCl, 1 mm EDT, 0.5% Nonidet P-40) containing 50 mm β-glycerophosphate,
Supplemental Materials and Methods Co-immunoprecipitation (Co-IP) assay Cells were lysed with NETN buffer (20 mm Tris-HCl, ph 8.0, 0 mm NaCl, 1 mm EDT, 0.5% Nonidet P-40) containing 50 mm β-glycerophosphate,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature09732 Supplementary Figure 1: Depletion of Fbw7 results in elevated Mcl-1 abundance. a, Total thymocytes from 8-wk-old Lck-Cre/Fbw7 +/fl (Control) or Lck-Cre/Fbw7 fl/fl (Fbw7 KO) mice
doi:10.1038/nature09732 Supplementary Figure 1: Depletion of Fbw7 results in elevated Mcl-1 abundance. a, Total thymocytes from 8-wk-old Lck-Cre/Fbw7 +/fl (Control) or Lck-Cre/Fbw7 fl/fl (Fbw7 KO) mice
Regulation of autophagic activity by ζ proteins associated with class III. phosphatidylinositol-3 kinase. Mercedes Pozuelo Rubio
 ONLINE SUPPORTING INFORMATION Regulation of autophagic activity by 14-3-3ζ proteins associated with class III phosphatidylinositol-3 kinase Mercedes Pozuelo Rubio entro Andaluz de Biología Molecular y
ONLINE SUPPORTING INFORMATION Regulation of autophagic activity by 14-3-3ζ proteins associated with class III phosphatidylinositol-3 kinase Mercedes Pozuelo Rubio entro Andaluz de Biología Molecular y
B. ADM: C. D. Apoptosis: 1.68% 2.99% 1.31% Figure.S1,Li et al. number. invaded cells. HuH7 BxPC-3 DLD-1.
 A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
No wash 2 Washes 2 Days ** ** IgG-bead phagocytosis (%)
 Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
supplementary information
 DOI: 10.1038/ncb1977 Figure S1 a. Immunofluorescence analysis of IFT20 localization in PBL costained with anti-β-tubulin antibodies. b. Immunofluorescence analysis of IFT20 localization in Jurkat cells,
DOI: 10.1038/ncb1977 Figure S1 a. Immunofluorescence analysis of IFT20 localization in PBL costained with anti-β-tubulin antibodies. b. Immunofluorescence analysis of IFT20 localization in Jurkat cells,
T H E J O U R N A L O F C E L L B I O L O G Y
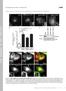 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in
 Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
Supplementary Figure 1 Pfn1, but not other Pfn isoforms are expressed in platelets. (a) RT-PCR of Pfn isoforms in control mouse platelets, Pfn1 -/- platelets and control heart. Expected band size for Pfn1
DCLK-immunopositive. Bars, 100 µm for B, 50 µm for C.
 Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Supplementary Figure S1. Characterization of rabbit polyclonal anti-dclk antibody. (A) Immunoblotting of COS7 cells transfected with DCLK1-GFP and DCLK2-GFP expression plasmids probed with anti-dclk antibody
Supplemental Data Supplementary Figure Legends and Scheme Figure S1.
 Supplemental Data Supplementary Figure Legends and Scheme Figure S1. UTK1 inhibits the second EGF-induced wave of lamellipodia formation in TT cells. A and B, EGF-induced lamellipodia formation in TT cells,
Supplemental Data Supplementary Figure Legends and Scheme Figure S1. UTK1 inhibits the second EGF-induced wave of lamellipodia formation in TT cells. A and B, EGF-induced lamellipodia formation in TT cells,
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 sirna and shrna mediated depletion of ATP7A results in loss of melanosomal ATP7A staining. a-h, sirna mediated ATP7A depletion. Immunofluorescence microscopy (IFM) analysis of ATP7A
Supplementary Figure 1 sirna and shrna mediated depletion of ATP7A results in loss of melanosomal ATP7A staining. a-h, sirna mediated ATP7A depletion. Immunofluorescence microscopy (IFM) analysis of ATP7A
In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier
 Supporting Information In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier Yang Song, Dan Du, Lei Li, Jun Xu, Prashanta Dutta *,, and Yuehe Lin *, School of Mechanical
Supporting Information In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier Yang Song, Dan Du, Lei Li, Jun Xu, Prashanta Dutta *,, and Yuehe Lin *, School of Mechanical
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
