A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos
|
|
|
- Bertha Fields
- 5 years ago
- Views:
Transcription
1 Development 126, (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos Gisèle A. Deblandre, Daniel A. Wettstein*, Naoko Koyano-Nakagawa and Chris Kintner Salk Institute for Biological Studies, PO Box 85800, San Diego, CA 92186, USA *Present address: Myriad Genetics, Inc., 320 Wakara Way, Salt Lake City, Utah 84108, USA Author for correspondence ( kintner@salk.edu) Accepted 11 August; published on WWW 6 October 1999 SUMMARY The skin of Xenopus embryos contains a population of specialized ciliated cells that are distributed in an evenly spaced pattern. Here we describe two successive steps that govern the differentiation and the generation of the spacing pattern of these ciliated cells. The first step occurs in the inner or sensorial layer of the non-neural ectoderm where a subset of cells are chosen to differentiate into ciliated-cell precursors. This choice is under the control of lateral inhibition mediated by a Suppressor of Hairless-dependent Notch signaling pathway, in which X-Delta-1 is the putative ligand driving the selection process, and a new Enhancerof-Split-related gene is an epidermal target of Notch signaling. Because nascent ciliated-cell precursors prevent neighboring cells from taking on the same fate, a scattered pattern of these precursors is generated within the deep layer of the non-neural ectoderm. Ciliated-cell precursors then intercalate into the outer layer of cells in the epidermis. We show that the intercalation event acts as a second step to regulate the spacing of the mature ciliated cells. We propose that the differentiation of the ciliated cells is not only regulated by Notch-mediated lateral inhibition, but is also an example where differentiation is coupled to the movement of cells from one cell layer to another. Key words: Xenopus, Ciliated cell, Spacing pattern, Lateral inhibition, Notch INTRODUCTION A common feature of epithelia in both plants and animals is that they contain specialized cells types, which are organized in ordered spatial patterns. Examples of such patterns include the trichomes (single-celled hairs) in the epidermis of Arabidopsis Thaliana, sensory organs with their associated bristles in insects, feather buds in the chicken skin and hair follicles in mouse skin. The common element in these examples is that the distance between the specialized cells tends to be maximized, giving rise to a so-called spacing pattern. Such spacing patterns are thought to arise developmentally by mechanisms involving lateral inhibition, a process in which one developing element produces an inhibitory signal that blocks the development of similar elements nearby (Doe et al., 1985; Wigglesworth, 1940). One form of lateral inhibition studied extensively involves the receptors of the Notch family and their ligands, which mediate a common mechanism for restricting the number of cells that will take on a particular fate from a group of competent cells (Artavanis-Tsakonas et al., 1995). In this mechanism, differentiating cells express a transmembrane ligand of Notch, for example Delta, which binds the Notch receptor on neighboring cells. Activation of Notch signaling leads, via the conserved transcription factor, Suppressor of Hairless [Su(H), also known as CBF-1 or lag-1], to the transcriptional activation of Notch target genes whose products block the progression of cells into the differentiated state (Wettstein et al., 1997 and references therein). The role of Notch-mediated lateral inhibition in determining the spacing of differentiated cells has been studied extensively in Drosophila where sensory organs form with a characteristic spacing pattern in the epidermis (Hartenstein and Posakony, 1989; Simpson, 1990). The position of sensory organs is determined by the bhlh transcription factors encoded by the Achaete- Scute complex (AS-C), whose expression endows dispersed clusters of cells in the ectoderm with the competence to form sensory organs precursors (SOP). Within the cluster, a nascent SOP expresses Delta, which activates Notch in adjacent cells. Activation of the Notch pathway ultimately inhibits the activity of the AS-C genes in the other cells of the cluster and prevents these cells from also differentiating into SOPs (Ghysen et al., 1993; Heitzler et al., 1996). Blocking Notch-mediated lateral inhibition results in an overproduction of SOPs within each cluster, but the spacing of the clusters within the epidermis is unaffected (Heitzler and Simpson, 1991). Thus, in this example, Notch signaling is used to control spacing at a local level, by preventing SOPs from forming adjacent to each other, but does not appear to act to generate spacing in a more global context. In vertebrates, the role of the Notch pathway in the patterning of non-neural epithelia has been examined during
2 4716 G. A. Deblandre and others Fig. 1. Ciliated cells are arranged in a regularly spaced pattern and are marked by the expression of an α-tubulin gene. (A,B) Scanning electromicrographs of the skin of a Xenopus embryo (stage 25) taken at a magnification of 200 (A) and 750 (B). Note the spacing pattern of cells with tufts of cilia in the outer layer of Xenopus skin (B, Arrow). (C) Xenopus embryo (stage 22) stained in whole mount using HRP immunohistochemistry with a mouse monoclonal antibody directed against acetylated α-tubulin (6-11B1). (D) Xenopus embryo (stage 25) double-labeled by in situ hybridization with the α-tubulin probe (light blue) followed by 6-11B1 antibody staining (brown). (E) Transverse section of the embryo shown in D, confirming that the same cells stain with both the α-tubulin probe and the 6-11B1 antibody (arrows). the formation of feather buds (Crowe and Niswander, 1998; Viallet et al., 1998) Feather buds form with a regular spacing pattern, and activation of the Notch pathway at a critical stage, abolishes bud formation. However, the spacing pattern of buds appears to be preset through a reciprocal interaction between the ectoderm and underlying mesoderm, involving growth factors (Jung et al., 1998; Noramly and Morgan, 1998, reviewed in Oro and Scott, 1998). In particular, BMPs are produced by the forming feather bud and acts as a long-range signal to restrict bud formation. In addition, recent evidence suggests that spacing of hair follicles in mammal skin may involve the Wnt signaling pathway (Gat et al., 1998). Thus, if Notch has a role in hair/feather follicle spacing, this role is more likely to be in controlling the size, polarity or the generation of cell types within the follicles, rather than their spacing within the epithelia. Elucidation of the mechanisms generating hair and feather spacing patterns is complicated by the fact that they are complex multicellular structures. By contrast, the ciliated cells in the Xenopus embryonic skin constitute a simple example of spacing pattern involving singlecelled epidermal appendages. Ciliated cells are arranged in the surface layer of the non-neural ectoderm in a strikingly ordered distribution (Fig. 1). Their function is enigmatic but, by beating together in the same direction, they could produce currents in the intravitelline medium, allowing the embryo to exchange oxygen or to distribute secreted products such as mucous or hatching enzymes. Mature ciliated cells are detected by stage 22, but by electromicroscopy, the precursors to the ciliated cells can be identified through numerous centrioles and precentriolar material accumulation as early as stage 13, when they appear to lie beneath the outer layer of cells (Billett and Gould, 1971; Steinman, 1968). The embryonic skin of Xenopus embryo is effectively composed of two layers of cells; the outer layer is made up of large columnar cells tightly adherent to each other while the inner, or sensorial layer is made up of smaller and loosely adherent cells. Tracing experiments have shown that ciliated cells originate from the inner layer: labeled grafts of inner cells produced labeled ciliated cells whereas labeled outer grafts were interspersed by non-labeled ciliated cells (Drysdale Fig. 2. Expression of α-tubulin in the skin of Xenopus embryos at different developmental stages. Expression of α-tubulin detected by whole-mount in situ hybridization on Xenopus embryos at stage 13 (A-C), stage 16 (D-F) and stage 20 (G-I). Dorsal views (A,D,G) and lateral views (B,E,H) show that α- tubulin expression occurs in the non-neural ectoderm (nne) and not in the neural plate (np). Expression is also absent from the cement gland (cg). (C,F,I) Sections of whole-mount-stained embryos show that cells expressing α-tubulin are located in the inner layer (parenthesis or arrow labeled i ) at stage 13, and in the outer layer (brackets labeled o ) at stage 16 and older, indicating that the ciliated cells first arise in the inner layer before stage 13 and then subsequently move into the outer layer.
3 Formation of the ciliated cells spacing pattern 4717 Fig. 3. X-Delta-1 is expressed in the ventral ectoderm in a punctate pattern that anticipates α-tubulin expression. (A-C) Xenopus embryo (stage 11) stained in whole mount for the expression of X-Delta-1 by in situ hybridization. View of the ventral side is shown in A, a lateral view in shown in B and a transverse section is shown in C. Note that X-Delta-1 is expressed by scattered cells within the inner layer of the ventral ectoderm (A,C), in addition to cells (B, arrowheads) that are localized to stripes within the neural plate where primary neurons will form (np). (D,E) α-tubulin expression is first detected by in situ hybridization at stage 12 in the ventral ectoderm (D). Lateral view shows that α-tubulin is expressed by scattered cells in the non-neural ectoderm (nne) but not in the neural plate (np). bl, blastopore. and Elinson, 1992). Thus, ciliated cells appear to form in the inner layer and to migrate into the outer layer at neurula stages. How these cells are generated and achieve their final spacing pattern is not known. Here, we examine the mechanisms that control the formation and spacing of the ciliated cells. We provide evidence that the mechanism responsible for their even distribution throughout the non-neural ectoderm of the embryo can be subdivided in two steps. The first step involves lateral inhibition via the Notch pathway, which restricts the number of cells in the sensorial layer that adopt the ciliated-cell fate. As this mechanism mostly precludes two precursors to be in direct contact, it presets the initial distribution pattern. The second step involves the migration of the ciliated-cell precursors from the inner to the outer layer of the epidermis. In itself, the intercalation into the tightly adherent layer of outer cells could prevent the migration of more than one inner cell per epithelial junction. Thus the ciliated cells distribution provides not only a new model system to study the lateral inhibition mechanism mediated by Notch and Delta in Xenopus embryos but also a simple example for studying the morphogenesis of an organized epithelial pattern. encoding a nuclear-localized form of β-galactosidase, along with 1-2 ng test RNAs (stated if otherwise) were injected into single blastomeres of albino embryos at the 2-cell stage as described previously (Coffman et al., 1993). For animal caps, 2-cell-stage Xenopus embryos were injected in the animal region of each blastomere with 1-2 ng of the indicated capped synthetic RNAs. MATERIALS AND METHODS Embryos and injections Embryos were obtained from Xenopus laevis adult frogs by hormone-induced egg laying and in vitro fertilization using standard methods. Xenopus embryos were staged according to Nieuwkoop and Faber (1967). Constructs cloned in pcs2+ vectors (Turner and Weintraub, 1994) were transcribed into capped RNA using SP6 RNA polymerase. Templates for generating RNA encoding Notch-ICD, X-Su(H)DBM and X-Delta1 Stu were obtained as described, respectively, in Chitnis et al. (1995), Coffman et al. (1993) and Wettstein et al. (1997). Templates for generating RNA encoding ESR-6e, ESR- 6e b, ESR-7 and ESR-7 b were obtained by linearization of their respective CS2 constructs by NotI. For in situ hybridization analysis, synthetic n-lacz RNA (20 pg), Fig. 4. Notch signaling restricts the number of α-tubulin-expressing cells. Albino embryos were injected at 2-cell stage in one blastomere with (A-C) nlacz RNA alone, (D-F) Notch-ICD and nlacz RNAs, (G-I) X-Su(H)-DBM and nlacz RNAs, or (J-L) X-Delta-1 Stu and nlacz RNAs. At stage (neurulae), the embryos were fixed, stained in whole mount with X-gal (lightblue reaction product) and then labeled by in situ hybridization for the expression of α-tubulin (dark purple). Left panels show a lateral view of the uninjected side, the middle panels a lateral view of the injected side, and the right panels show a dorsal view. Note that Notch-ICD suppresses α-tubulin expression in 93% of injected embryos (57 embryos in four representative experiments) while the density of α-tubulin-expressing cells increases in embryos injected with X-Su(H)-DBM (55/64 injected embryos) or with X- Delta-1 Stu (64/80 injected embryos) in three independent experiments.
4 4718 G. A. Deblandre and others Whole-mount in situ hybridization, immunostainings and histology Whole-mount in situ hybridization of Xenopus embryos was performed according to Harland (1991) with modifications described by Knecht et al. (1995) using digoxigenin-labeled antisense RNA probes for X-Delta-1 (Chitnis et al., 1995), Xenopus α-tubulin and ESR-6e (full-length sequences were subcloned in pbs-sk, linearized, respectively, by NotI or BamHI and transcribed by T7 RNA polymerase). For detection of acetylated α-tubulin, embryos were stained with the monoclonal antibody (Ab) 6-11B1 (Sigma) as described (Bradley et al., 1998). Prior to in situ hybridization or immunostaining, injected embryos were stained for β-galactosidase activity with X-gal or Magenta-gal (Biosynth) to localize the tracer. Embryos to be sectioned were embedded in Paraplast, serially sectioned into 10 µm thick sections, dewaxed, stained with eosin and finally photographed under a phase-contrast microscope. Animal caps experiments and RNase protection assays (RPA) Animal caps were dissected at stage 9 and cultured on agarose-coated Petri dishes in 0.5 MMR containing gentamycin until sibling controls reached the stage noted. In experiments where the two ectodermal layers were separated, outer ectodermal layers of whole stage 9 embryos animal caps were removed manually and set aside on agarose coated culture dishes in 0.5 MMR. The inner layer of the animal cap was then cut off from the rest of the embryo and cultured likewise in another dish. The inner layer preparations were visually checked for absence of contamination by pigmented outer layer cells. RNA was isolated and analyzed by RNase protection assay using 32 P-labeled antisense RNA probes as previously described (Kintner and Dodd, 1991; Kintner and Melton, 1987). The template used for the α-tubulin probe corresponds to the EcoRV-HindIII fragment contained in the second half of the open reading frame, while that for X-Delta-1 has been described previously (Chitnis and Kintner, 1996). ESR-6e probe corresponds to the fragment of the bhlh domain initially isolated by RT-PCR, which was subcloned between the XhoI and BamHI sites in pbs-sk(stratagene). RNA samples isolated from 10 animal caps, or from 2 animal caps for α-tubulin assays, were analyzed simultaneously along with the EF1-α probe to control for sample recovery during the assay. Quantification was carried out on a PhosphorImager (Molecular Dynamics) and, for each lane, specific band intensities were normalized to the amount of EF1-α RNA. Isolation of cdna encoding α-tubulin, ESR-6e and ESR-7 Α cdna encoding α-tubulin was isolated during a screen for genes that are induced by neurogenin (XNGN-1). A cdna library in λzap was constructed using poly(a) + RNA prepared from animal caps that were isolated from embryos injected with noggin (Lamb et al., 1993) and XNGN-1 RNAs. Upon differential screening, putative positive clones were sequenced and tested for expression by whole-mount in situ hybridization. The cdna encoding α-tubulin proved to be a false positive, which nonetheless marked the formation of ciliated cells. Novel sequences encoding WRPW-bHLH proteins were isolated by RT-PCR as described in Jen et al. (1999) using RNA prepared from animal caps isolated from embryos injected with RNA encoding noggin and Notch-ICD. Products of the RT-PCR reaction (135 bp) were subcloned in pbs-sk and sequenced. The novel sequences obtained by RT-PCR were then used as probes to screen a λgt10 stage 17 cdna library (Kintner and Melton, 1987) yielding full-length cdna clones that encode ESR-6e and ESR-7. Nucleotide sequences for the cdnas encoding ESR-6e and ESR-7 have been submitted to GenBank under the accession numbers AF and AF146088, respectively. Constructs and mutagenesis of ESR genes λgt10 cdnas inserts were removed by EcoRI digestion, subcloned into the EcoRI site of pbs-sk (Stratagene) and used as templates for the generation of digoxigenin-labeled RNA probes. For generating synthetic messenger RNA in vitro, the coding regions of ESR-6e and ESR-7 were amplified by PCR using the following forward/reverse primers: ESR-6e: TCAGAATTCACCATGGAGAGAAAG/TCACTCGAG- ATTAAGGAGGTGA ESR-7: TCAGAATTCACCATGGCACCCA/TCACTCGAGATT- ACCACGGCC. The PCR products were digested with EcoRI and XhoI and inserted between the EcoRI and XhoI sites in the pcs2 polylinker. ESR-6e b and ESR-7 b encode mutant forms of the respective ESR cdnas that have been obtained by substitution of amino acids in the basic region. Oligonucleotide-directed mutagenesis (Kunkel, 1985) was performed using the following sense primers: ESR-6e: GAGAGAAAGCCAGTGATTCGGGAGATGGAAG- AAGATGATATCGACAACAGCATT ESR-7: ACGAAAGCCAGTTGTGCGGGAGATGGAAGAG- GATGACATTGACAGCAGCAT RESULTS Isolation of a new marker of the ciliated cells and their precursors As observed by scanning electromicroscopy performed at any stage after stage 22, the epidermis of Xenopus embryos contains specialized cells with tufts of cilia that are arranged in a regular spacing pattern (Fig. 1A,B). From stage 20 onwards, the ciliated cells can be detected using the antibody 6-11B1, which reacts with acetylated α-tubulin (Fig. 1C), a stable form of α-tubulin found in microtubules (Chu and Klymkowsky, 1989). Staining with 6-11B1 occurs only when the ciliated cells begin assembling considerable amounts of microtubules required for cilia growth, thus making it a relatively late marker for ciliated cells. However, while screening a cdna library for genes expressed during neurogenesis, we fortuitously isolated an α-tubulin cdna, which is expressed by evenly spaced cells in the non-neural ectoderm at earlier stages (Fig. 2). The cells that stain with the α-tubulin probe also stain with the 6-11B1 antibody in doublelabeled embryos, indicating that the ciliated cells express α- tubulin (Fig. 1D,E). In order to establish a time course for α-tubulin expression, embryos at different developmental stages were stained with α-tubulin probe using whole-mount in situ hybridization. α- tubulin expression is first detected in late gastrula-stage embryos (stage 12) as a diffuse but scattered pattern of staining in the animal pole ectoderm (Fig. 3D,E). By early neurula stages (stage 13), expression of α-tubulin is clearly restricted to the non-neural ectoderm (Fig. 2A-C). Between stages 13 and 16, the α-tubulin mrna accumulates dramatically in a subset of cells within the non-neural ectoderm and, from stage 16 onwards, the α-tubulin-expressing cells are clearly organized in a spacing pattern (Fig. 2D-I). The neural plate and cement gland are devoid of α-tubulin-positive cells at all stages. α- tubulin expression stays on at tadpole stages (stage 30) and remains restricted to cells within the epidermis with the characteristic spacing pattern of ciliated cells (data not shown). The ciliated cells originate from the inner layer of the ectoderm and intercalate to the surface Since the α-tubulin marker allowed us to detect the cells that eventually become ciliated much earlier than the 6-11B1 staining, we were able to follow the differentiation of these cells, from gastrula stages when they are first generated, to
5 Formation of the ciliated cells spacing pattern 4719 early tadpole stages when they are completely differentiated as ciliated cells. In tissue sections of stained embryos, the α- tubulin-expressing cells are initially located only in the sensorial layer of the ectoderm (Fig. 2C). From stage 16 onwards, the α-tubulin-expressing cells are located exclusively in the superficial layer (Fig. 2F,I), suggesting that they have intercalated from the lower layer outwards. By stage 20, the α- tubulin-expressing cells have completed their migration (Fig. 2I) and, by stage 23, they are capped by the characteristic tuft of cilia (Fig. 1E). These observations indicate that the ciliated cells first arise in the deep layer at gastrula stages, intercalate into the superficial layer around stage 15, and then fully differentiate by stage 23. A lateral inhibition process restricts the number of ciliated-cell precursors The results obtained with the α-tubulin marker indicate that only a subset of the inner layer cells appear to be committed to the ciliated-cell fate. Furthermore, staining for α-tubulin expression performed around stage (Fig. 2A-C) indicated that, even at these early stages, the ciliated-cell precursors are not adjacent to each other, suggesting that their formation is regulated by cell-cell interactions that restrict the number of these precursors. Given the ubiquitous role of the Notch pathway in selecting out specialized cell types from a field of equipotential cells, we asked whether the specification of the ciliated-cell fate is restricted by lateral inhibition as mediated by X-Notch-1 and its ligand X-Delta-1. To examine the role of the Notch pathway in the specification of ciliated cells, we first examined the expression of X-Delta-1 using whole-mount in situ hybridization at stages when the ciliated-cell precursors are likely to form (stages 11-12). This analysis showed that a population of cells located in the non-neural ectoderm, opposite to the blastopore, expresses X-Delta-1 in a scattered pattern that prefigures the cells that will express α-tubulin (Fig. 3A,B). In addition, tissue sections demonstrated that these X-Delta-1-positive cells are located in the inner layer of the ectoderm where the α-tubulin-expressing cells are apparently generated (Fig. 3C). At stages 12-13, when α-tubulin expression first occurs (Fig. 3D,E), X-Delta-1 expression is downregulated in the non-neural ectoderm. By stage 13, X-Delta-1 is no longer detectable outside of the neural plate (data not shown). Thus, X-Delta-1 expression occurs transiently within the sensorial layer of the non-neural ectoderm and corresponds to the time in development when the ciliated-cell precursors are likely to form. To assess whether the formation of the ciliated-cell precursors is regulated by the Notch pathway, we used several reagents that have proven effective at activating or inhibiting Notch signaling in primary neurogenesis (Chitnis et al., 1995; Wettstein et al., 1997). Activation of the Notch pathway can be achieved by overexpressing the intracellular domain of Notch (Notch-ICD), which constitutively translocates to the nuclei where, in association with Suppressor of Hairless [X-Su(H)], it transactivates Notch target genes. Expressing Notch-ICD in embryos blocks the formation of primary neurons. Alternatively, Notch signaling can be inhibited by expressing a DNA binding mutant of Su(H), X-Su(H)-DBM, or by expressing a form of the X-Delta-1 ligand that lacks an intracellular domain, called X-Delta-1 Stu. Expressing X- Su(H)-DBM or X-Delta-1 Stu in embryos by RNA injection causes more primary neurons to form than normal. Thus, we injected RNA transcripts encoding Notch-ICD, X-Su(H)-DBM and X-Delta-1 Stu into one blastomere of 2-cell-stage embryos, along with a tracer RNA, nlacz (see Materials and Methods) and tested for α-tubulin expression. Injecting embryos with just nlacz RNA, (Fig. 4A-C) did not affect the density or the distribution of the α-tubulin-expressing cells. By contrast, injecting embryos with RNA encoding the constitutively active form of X-Notch1, Notch-ICD, resulted in a complete loss of the α-tubulin-expressing cells, as predicted if Notch signaling negatively regulates the formation of ciliated-cell precursors (Fig. 4D-F). The RNA encoding Notch- ICD inhibited the formation of α-tubulin-expressing cells over a range of 2 ng to 125 pg of injected RNA per embryo. Injection of RNAs encoding the molecules that block Notch signaling, X-Su(H)-DBM or X-Delta-1 Stu, led to a dramatic increase in the density of the ciliated cells precursors (Fig. 4G- L) so that the α-tubulin-expressing cells filled the whole injected region of the inner ectodermal layer and were no longer separated by non-expressing cells. In some embryos, more severe phenotypes were observed where the ectodermal cell layer was thickened and filled by several layers of α- tubulin-expressing cells (Fig. 8C). Thus, these results indicate that the number of ciliated-cell precursors generated during gastrula stages is regulated by the Su(H)-dependent Notch pathway. Previous studies have shown that overexpressing the wildtype form of X-Delta-1 in the neural plate suppresses the formation of primary neurons, as expected for a ligand that activates Notch signaling. However, when X-Delta-1 was overexpressed in the non-neural ectoderm, it did not inhibit the ciliated-cell fate but, on the contrary, caused an overproduction of α-tubulin cells with phenotypes ressembling those caused by X-Delta-1 Stu RNA injection (not shown). The penetrance of the X-Delta-1 phenotype was weaker: only 45% of injected embryos on a total of 94 (in 4 independent experiments) displayed the phenotype compared to 80% for X-Delta-1 Stu. While this result is at odds with the idea that X-Delta-1 acts as an inhibitory ligand during the formation of the α-tubulinexpressing cells, it is reminiscent of similar results in Drosophila, where overexpressing Delta usually leads to a reduction of Notch signaling, suggesting a dominant negative effect (see Discussion). Lateral inhibition regulates X-Delta-1 expression within the non-neural ectoderm In Drosophila, expression of Delta is positively regulated by the proneural genes, whose activity, in turn, is negatively regulated by the Notch pathway. In principle, therefore, the levels of Delta expression between cells undergoing lateral inhibition is modulated so that Delta expression reaches high levels in differentiating cells and low levels in cells whose differentiation is blocked by the Notch pathway. To examine whether X-Delta-1 expression is similarly modulated during the generation of ciliated-cell precursors, we examined the expression of X-Delta-1 in embryos where the Notch pathway had been activated or inhibited. As shown in Fig. 5, when embryos were injected with Notch-ICD RNA, the expression of X-Delta-1 was suppressed, whereas injecting RNA encoding X-Su(H)-DBM produced the opposite effect: a large increase in the number of X-Delta-1-expressing cells. To confirm these
6 4720 G. A. Deblandre and others Fig. 5. Lateral inhibition in the non-neural ectoderm feeds back on X-Delta-1 expression. (A-C) The ventral ectoderm of albinos embryos (stage 11) that were uninjected (A) or injected in one blastomere at the 2-cell stage with RNA encoding Notch-ICD (B) or X-Su(H)-DBM (C) along with nlacz RNA as a tracer. Embryos were stained with X-Gal (sky blue reaction product) or for the expression of X-Delta-1 using whole-mount in situ hybridization (dark purple). Note that the density of X-Delta-1 expression increases when Notch signaling is blocked (C) and decreases when Notch signaling is increased (B). (D,E) Isolated ectoderm was assayed for the levels of α-tubulin (α-tub) and X-Delta-1 RNAs by RPA. Ectoderm was isolated at stage 9/10 from embryos that were uninjected (uninj.) or injected at 2-cell stage in both blastomeres with RNA encoding Notch-ICD or X-Su(H)-DBM. RNA was extracted at stage 18 or 12 and assayed simultaneously for the levels of α-tubulin and EF1-α RNA, or for X-Delta-1 and EF1-α RNA, respectively. Note that increased Notch signaling (Notch-ICD injection) decreases, while decreased Notch signaling (X-Su(H)-DBM injection) increases the levels of X-Delta-1 and α-tubulin when normalized to recovery of RNA using the levels of EF1-α RNA. RNA from whole uninjected embryos (w. emb.) was used as positive control. Fig. 6. ESR-6e encodes a WRPW-bHLH protein expressed epidermally and activated by the Notch pathway. (A) ESR-6e encodes a WRPW-bHLH protein related to other Xenopus ESR genes that are activated by the Notch pathway. ESR-1 and ESR-7 are expressed in the developing nervous system, while ESR-5 is expressed in the paraxial mesoderm during segmentation. Percentages of similarity between the bhlh domains of ESR-6e and other ESR factors identified in Xenopus are given as well as the overall similarity for the full-length proteins. (B) Expression of ESR-6e RNA in stage 14 (neural plate) Xenopus embryos as detected by whole-mount in situ hybridization. Note that ESR-6e expression is mostly localised to the non-neural ectoderm as shown by the white dashed line marking the border of the neural plate. A high level of expression is also observed in the cement gland (white arrowhead) and a low level in the central neural plate (black arrowhead). (C) Total RNA extracted from embryos at the indicated stages was assayed by RPA for the levels of ESR-6e RNA and EF1-α RNA as a loading control. The levels of ESR-6e at different stages are plotted in a bar graph after normalizing to the levels of EF1-α RNA. EF1-α RNA is first transcribed at the mid-blastula transition, reaching steady levels at stage 12. Thus, the levels of ESR-6e RNA are likely to be overestimated after normalizing to the levels of EF1-α RNA at stage 8 and 10. (D) Notch-ICD RNA was coinjected with nlacz RNA in one blastomere of 2-cell-stage embryos. At stage 14, the embryos were stained with X-gal (light blue) and for ESR-6e expression using in situ hybridization (dark blue). Note that Notch-ICD RNA induces high levels of ESR-6e expression but only in the non-neural ectoderm in 48 injected embryos on a total of 54. In this example, the Notch-ICD-injected area (circled by the black dashed line) extends into the neural plate but ESR-6e overexpression stays confined to the prospective epidermis (white dashed line) as in B. Note also that the embryo shown in D has been stained for less time than the one in B, as indicated by the reduced signal from the basal expression of ESR-6e. (E,F) Transverse sections of a Notch-ICD-injected embryo as in D. (G) RNase protection assay to measure the levels of ESR-6e RNA in ectoderm isolated from embryos injected with RNA encoding Notch-ICD or X-Su(H)-DBM as described in the legend to Figure 5. RNA was extracted at stage 11 from intact ectoderm (w. caps) or from the inner layer (inner) or outer layer (outer) after manual separation. Note that Notch-ICD induces higher levels of ESR-6e expression in both layers. X-Su(H)-DBM also increases the levels of ESR-6e in the inner layer but not in the outer layer.
7 Formation of the ciliated cells spacing pattern 4721 results, we also measured the levels of X-Delta-1 mrna in an animal cap assay. Ectodermal explants dissected out before mid-gastrula stages form atypical epidermis only and no neural ectoderm. Although this isolated tissue is not properly organized into two layers, it nevertheless develops ciliated cells and express epidermal markers such as epi and XK81 (Akers et al., 1986; Miyatani et al., 1986; Wilson and Hemmati- Brivanlou, 1995; Xu et al., 1997). Embryos were injected at 2- cell stage with 2 ng of Notch-ICD or X-Su(H)-DBM RNA in the animal pole of each blastomere. Animal caps were dissected at stage 8 and left to develop until sibling embryos reached stage 11, when total RNA was extracted. RNase protection assays (RPA) with X-Delta-1 probe confirmed that the expression of Notch-ICD blocks X-Delta-1 expression while expression of X-Su(H)-DBM increases it (Fig, 5E). We also verified that the variations of X-Delta-1 mrna levels correlated with those of α-tubulin mrna as measured by RPA (Fig. 5D). These complementary results are a strong indication that Notch signaling effectively regulates X-Delta-1 expression during the process of selecting out the ciliated-cell precursors. Furthermore, these results indicate that Notch signaling regulates the activity of an unknown upstream factor that not only promotes the differentiation of ciliated-cell precursors, but also the expression of X-Delta-1. A novel bhlh-wrpw transcription factor activated by the Notch pathway In many cases where Notch acts during Drosophila development, its downstream signaling activates genes in the E(spl)-C. Many of them encode bhlh proteins that share several characteristic structural features, namely a conserved proline in the basic region and a C-terminal WRPW tetrapeptide necessary for interaction with the co-repressor Groucho (Bailey and Posakony, 1995). To further examine how the Notch pathway might regulate the formation of α-tubulinexpressing cells, we used RT-PCR to amplify sequences that encode WRPW-bHLH proteins from RNA isolated from Notch-ICD-expressing animal caps. This approach led to the identification of a cdna that encodes a novel WRPW-bHLH protein with significant homology to other Enhancer of Split Related factors (ESR) known to act downstream of Notch signaling in Xenopus. Based on this homology, and its regulation by the Notch pathway (see below), the new Xenopus protein was termed ESR-6e (Xenopus Enhancer of Split Related-6 epidermal). Compared to the other ESR proteins identified so far, ESR-6e shares the highest level of similarity with ESR-1 (D. L. Turner, personal communication), a WRPW-bHLH protein expressed in the neural plate, with 76% homology between their bhlh domains (Fig. 6A). The expression of ESR-6e was localized in early Xenopus embryos by whole-mount in situ hybridization at neurula stages, when ESR-6e is strongly expressed in the non-neural ectoderm, including the cement gland. By contrast, ESR-6e expression was undetectable in the neural plate except for extremely weak expression in the medial neural plate (Fig. 6B). In addition, ESR-6e staining in the non-neural ectoderm is mostly localized to the outer layer of cells (Fig. 6F) where it is relatively uniform (Fig. 6B.). Even though ESR-6e expression could be detected weakly in the inner layer of the non-neural ectoderm (Fig. 6F), whole-mount in situ hybridization proved not to be sensitive enough to detect ESR-6e expression before neurula stages or to allow the vizualisation of a pattern of expression that could be related to X-Delta-1 or early α-tubulin expression patterns. With the aim to establish if ESR-6e could play a role during the formation of α-tubulin-expressing cells, we used a more sensitive method, RPA, to detect ESR-6e RNA expression in Fig. 7. XESR-6e restricts the number of ciliated cell precursors. (A-D) One blastomere of 2-cell-stage albino embryos was injected either with 0.5 ng of ESR-6e RNA or 2 ng of ESR-6e b RNA along with nlacz RNA as a tracer. At stage 19-20, the injected embryos were fixed and stained with X-gal (light blue) and α-tubulin expression by in situ hybridization (dark blue). Embryos are oriented with anterior to the left with injected sides shown in B and D and the uninjected sides shown in A and C. (A,B) Injection of ESR-6e RNA suppressed the formation of α- tubulin-expressing cells in 90% of the embryos that survived the injection and developed normally (23 embryos in two independent experiments) whereas (C,D) injecting RNA encoding a DNA-binding mutant, ESR-6e b, produced an increase in α-tubulin-expressing cells in 90% of 48 injected embryos. (E) RPA on ectoderm isolated from embryos injected with RNA encoding ESR-6e and ESR-6e b (0.5 and 2 ng respectively) as described in Fig. 5. RNA was extracted from stage 11 animal caps for assessing X-Delta-1 expression and at stage for α-tubulin. Note that ESR-6e decreases the levels of both X-Delta-1 and α- tubulin RNA while ESR-6e b increases their levels. (F) RPA of RNA isolated from ectodermal caps of embryos injected with RNA encoding wild-type (0.5 ng) and basic domain mutant forms of ESR-6e and ESR-7 (2 ng) as described above. Note that injection of RNA encoding ESR- 6e or ESR-6e b leads to a decrease or increase, respectively, in the levels of α-tubulin RNA. Conversely, RNA encoding ESR-7 causes a decrease in the levels of α-tubulin RNA while RNA encoding ESR-7 b has no apparent effect.
8 4722 G. A. Deblandre and others embryos and found that expression could be detected soon after the mid-blastula transition, with levels peaking at stage 10 to 12 and then decreasing through tadpole stages (Fig. 6C). Moreover, using RPA, we could detect ESR-6e RNA at stage in both the inner and outer ectodermal layers when assayed separately after manual separation (Fig. 6G). Thus, RPA results indicate that ESR-6e RNA is also expressed at earlier stages, within the inner layer, at the time and place where the ciliated-cell precursors form. ESR-6e is regulated by the Notch pathway We next examined whether ESR-6e expression in the ectoderm is regulated by the Notch pathway. In embryos injected with RNA encoding an activated form of the receptor, Notch-ICD, ESR-6e expression was dramatically increased, as expected for a Notch target gene (Fig. 6D-G). Strikingly, ESR-6e expression increased markedly in the non-neural ectoderm but not detectably in the neural ectoderm, even when Notch signaling was activated in a large region of the embryo extending into the neural plate (Fig. 6D). By comparison, other Notch target genes, such as ESR-1 or ESR-7, respond to activated Notch by upregulating in both the non-neural and neural ectoderm (data not shown). Transverse sections demonstrated that ESR-6e responded to Notch-ICD RNA injection by increasing in both the outer layer, as well as in the inner layer, where expression is normally more difficult to detect (Fig. 6E,F). RPA confirmed that the expression of ESR-6e was induced in both the inner and outer cell layer of ectoderm isolated from embryos injected with Notch-ICD RNA (Fig. 6G). Thus, these results indicate that the expression of ESR-6e can be promoted by the Notch signaling pathway. To determine if Notch signaling via Su(H) is necessary for ESR-6e expression, we also assayed, at stage 11-12, the levels of ESR-6e in the inner and outer layer of isolated ectoderm from embryos injected with X-Su(H)-DBM RNA. The results show that expression of X-Su(H)-DBM did not affect much the levels of ESR-6e RNA in intact ectoderm (Fig. 6G), which reflects the results obtained with the outer layer as a significant portion of ESR-6e expression is accounted by expression in the outer layer (Fig.6E). However, in the deep cell layer, the expression of X-Su(H)-DBM appeared to increase the levels of ESR-6e RNA. Similar results have been obtained with the E(spl)-C genes that are regulated by the Notch pathway in Drosophila (see Discussion), suggesting that the expression of ESR-6e is not only regulated by Notch signaling but by other factors as well. ESR-6e functions to restrict the number of ciliated precursors If ESR-6e acts downstream of Notch to restrict the number of ciliated cells, then altering the activity of ESR-6e should change the number of α-tubulin-expressing cells that form. To address this point, we increased ESR-6e activity by injecting ESR-6e RNA into embryos. Conversely in order to decrease ESR-6e activity, we designed a potential dominant negative mutant (ESR-6e b) in which key residues of the basic domain were modified ( 8 EKMRRDRIN 16 >REMEEDDID), producing a form that should not bind DNA. The design of the mutant was based on the premise that these proteins act primarily as dimers, and that a DNA-binding mutant subunit would inactivate endogenous wild-type molecules. Injection of RNA encoding ESR-6e or ESR-6e b into embryos produced a marked decrease and increase, respectively, in the number of α-tubulin-expressing cells as predicted if ESR-6e acts downstream of Notch to regulate the formation of ciliated-cell precursors (Fig. 7A-D). As a specificity control for these experiments, we also injected RNA coding for another ESR family member, ESR-7, which is activated by the Notch pathway but is expressed in the neural plate in the three domains where the primary neurons form, and not in the non-neural ectoderm (data not shown). The bhlh domains of ESR-7 and ESR-6e share 70% identity (Fig. 6A). We therefore exploited the fact that these two very related WRPW-bHLH proteins, which are both activated by the Notch pathway, have different expression domains to test the specificity of the ESR-6e b mutant. In these experiments, RNAs encoding wild-type and basic domain mutants of ESR-6e and ESR-7 were injected into embryos. Ectodermal caps were removed at stage 9 and assayed for expression of X-Delta-1 at stage 11 and α-tubulin at stage 19 by RPA. Injection of either ESR-6e or ESR-7 RNA led to a decrease in the levels of X-Delta-1 and α-tubulin RNA (Fig. 7E,F) in animal caps, mirroring the effects seen when the Notch pathway is activated by ICD. Significantly, injection of ESR-6e b RNA caused an increase in the expression levels of X-Delta-1 and α-tubulin RNA (Fig. 7E,F) while injection of ESR-7 b RNA did not (Fig. 7F). Moreover, co-injecting ESR- 6e b RNA partially rescued the loss of α-tubulin expression caused by ESR-6e but not by ESR-7 (Fig. 7F). These results establish ESR-6e b as a specific dominant negative form of ESR-6e and argue strongly in favor of ESR-6e acting downstream of X-Notch-1 in the lateral inhibition process that restricts the number of ciliated cells precursors. The intercalation of ciliated cells precursors is a second step responsible for establishment of the final spacing pattern The results described above indicate that the α-tubulinexpressing cells are initially generated in a fine-grained pattern as a result of lateral inhibition. As development proceeds and ciliated-cell precursors intercalate into the outer layer and differentiate, the pattern becomes increasingly organized into a spacing pattern. For example, in a majority of embryos, the α-tubulin-expressing cells are produced in a higher number anteriorly: they are denser and smaller at the limit of the open head folds and around the cement gland (Fig. 2E). However, by stage 20, the distribution of the ciliated-cell precursors appears more homogeneous throughout the whole embryo even if their density is often higher on the forming head (Fig. 2G,H). This observation suggests that even if lateral inhibition presets a salt and pepper distribution pattern of ciliated-cell precursors, other mechanisms contribute to the final organization of the ciliated-cell pattern in later embryos. If this is indeed the case, when lateral inhibition fails, a certain degree of organization in the pattern of the ciliated cells should remain. To test this idea, we blocked lateral inhibition in the nonneural ectoderm by injecting embryos with RNA encoding X- Su(H)-DBM. When an aliquot of these embryos were processed at stage 16, the density of α-tubulin-expressing cells on the injected side was increased to a varying degree as shown above (Fig. 8A-C). In embryos with a mild phenotype, α- tubulin-expressing cells were located adjacent to each other in
9 Formation of the ciliated cells spacing pattern 4723 the sensorial layer while, in the more severe cases, the α- tubulin-expressing cells were not only adjacent, but formed several layers in thickness (Fig. 8C). We then examined how the mild and severe increases in α-tubulin-expressing cells affected the final spacing pattern of the ciliated cells later in development, by processing the remaining embryos around stage 25. These embryos were fixed, stained for the tracer distribution with Magenta-gal (Fig. 8, magenta staining) and double-labeled with the α-tubulin probe by in situ hybridization (Fig. 8, light-blue staining) and with the 6-11B1 antibody to detect the differentiated ciliated cells (Fig. 8, brown staining). In embryos with the mild phenotype (Fig. 8D-H), all the α-tubulin-expressing cells appeared to have differentiated in mature ciliated cells by stage 25, as marked by 6-11B1 staining (Fig. 8F,G). These differentiated ciliated cells were still organized into a spacing pattern in the outer layer, but their density was approximately doubled, compared to the uninjected side (Fig. 8F-H). This result indicates that increasing the number of ciliated-cell precursors increases the density of differentiated ciliated cells. In embryos with a severe phenotype, the number of α-tubulin-expressing cells increased to the point where the α-tubulin staining in whole mount appeared relatively uniform in the injected area compared to uninjected area or to embryos with milder phenotypes (compare Fig. 8I and D). The spacing of these cells in the outer layer was difficult to assess in whole mount because the brown color corresponding to 6-11B1 Ab staining was obscured due to the high overexpression of α-tubulin (Fig. 8I). Nonetheless, some dark-blue cells (blue and brown stainings combined) emerged from the homogeneous light-blue staining in a regular pattern (Fig. 8I arrow and Fig. 8L), suggesting that these cells were still in a spacing pattern, despite the overproduction of ciliated-cells precursors. This interpretation was confirmed when these regions were examined in tissue section. In these sections, the inner layer of the ectoderm, which is normally devoid of α-tubulin-expressing cells at this stage, was filled with cells that not only stained with α-tubulin, but also mostly stained for the 6-11B1 antibody (Fig. 8K,N arrowheads). This overabundance of α-tubulin-expressing cells accounted for the relatively uniform α-tubulin staining observed when these severely affected embryos were examined in whole mount (compare Fig. 8D,I and F,L). These sections also confirmed that, in the surface layer of the skin, differentiated ciliated cells were surrounded by non-ciliated cells (Fig. 8N asterisks) and organized in a spacing pattern. Thus, this result indicates that when lateral inhibition is blocked and ciliated-cell precursors are produced in excess, they still form a normal spacing pattern in the outer layer, while excess precursors are left in the inner layer where they inappropriately differentiate. This inappropriate differentiation apparently explains the defects in the morphology of the epidermis of severely affected embryos (Fig. 8I,K). Finally, we note that, in these embryos, some of the excess inner ciliated cells appeared to be undergoing intercalation at stage 25 (Fig. 8N arrows), suggesting that intercalation can occur over a protracted period of time, as the embryo grows and the epidermis expands. DISCUSSION Here we describe two successive steps that govern the spacing pattern of ciliated cells in the skin of Xenopus embryos (Fig. 9). The first step occurs in the inner layer of the non-neural ectoderm where a subset of cells are chosen to differentiate into ciliated-cell precursors. This choice is under control of lateral inhibition mediated by Notch signaling, in which X-Delta-1 is the putative ligand driving the selection process and a new ESR gene is an epidermal target of Notch signaling. The second step involves intercalation of the ciliated precursors into the outer ectodermal layer when they move outwards to reach their definitive position at the surface of the embryonic skin. The intercalation imposes a restriction on every differentiated ciliated cell to be surrounded by non-ciliated cells, thus generating the final spacing pattern. A mechanism of lateral inhibition in the non-neural ectoderm of Xenopus embryos α-tubulin is an early marker of the ciliated cells that has allowed us to follow these cells along their differentiation path. We have shown unambiguously that ciliated-cell precursors arise from a subset of cells in the inner layer of the non-neural ectoderm as suggested earlier from transplantation studies (Drysdale and Elinson, 1992). Several lines of evidence presented here indicate that the ciliated-cell precursors are selected by a lateral inhibition mechanism mediated by Notch. First, expression of X-Delta-1 is transiently detected in a scattered pattern in the sensorial layer of the non-neural ectoderm with a peak of expression occurring shortly before the onset of α-tubulin expression (stage 11). This relationship between the expression of X-Delta-1 and α-tubulin parallels that seen between Delta and neuronal markers in developing neurons in the spinal cord or in the otic epithelium (Adam et al., 1998; Chitnis et al., 1995; Ericson et al., 1992; Haddon et al., 1998; Henrique et al., 1995; Memberg and Hall, 1995; Tsuchida et al., 1994). Thus it is likely that the cells expressing the highest level of X-Delta-1 at the time of the specification are inner layer cells that will adopt the ciliated-cell fate while repressing their neighbours differentiation. This interpretation is supported by the results of functional experiments in which an activated form of X-Notch-1 (Notch-ICD) suppresses the formation of ciliated cells while blocking Notch signaling using the dominant-negative form of X-Su(H), X-Su(H)-DBM, increases dramatically the number of α-tubulin-expressing cells. In addition, the activity of the Notch pathway during this process appears to be under control of a negative feedback loop. Expressing the activated form of X-Notch-1 or overexpressing a downstream target like ESR-6e led to X- Delta-1 downregulation. Conversely, blocking Notch signaling or its downstream target with the dominant negative forms of X-Su(H) or ESR-6e, respectively, caused X-Delta-1 upregulation. Thus, these observations fit the proposed model of lateral inhibition in which differentiating cells not only inhibit other cells from differentiating, but the selection of the cells that will differentiate depends on a negative feedback loop that generates winners and losers during lateral inhibition (Ghysen et al., 1993; Heitzler et al., 1996). Unexpectedly, X-Delta-1 overexpression led to an increase in α-tubulin-expressing cells in nearly half of the injected embryos, which reproduces, with a lower penetrance, the dominant negative phenotype as obtained by injection of X- Delta-1 Stu RNA. Overexpressing X-Delta-1 in the non-neural
10 4724 G. A. Deblandre and others Fig. 8. Spacing of ciliated cells is imposed by intercalation. Notch signaling was blocked in the ectoderm of Xenopus embryos by injecting 2 ng of X-Su(H)-DBM RNA into two blastomeres of 4-cell-stage embryos, along with nlacz RNA as a tracer. (A-C) At stage 17, a sample of embryos were fixed and the distribution and density of the α-tubulin-expressing cells was assessed by in situ hybridization with α-tubulin probe (dark blue) after detection of the tracer by X-gal staining (light blue). (C) A section through the embryo shown in A,B illustrates that blocking Notch signaling can result in several layers of α-tubulin-expressing cells. (B) The white dashed line indicates an area of the uninjected side of the embryo that has been populated by cells descending from the injected blastomeres as a result of cell mixing. (D-N) The remaining embryos were fixed at stage 25. In these embryos, the distribution of injected RNA was measured by staining with Magenta-gal (magenta), the density of α-tubulin-expressing cells was measured by in situ hybridization using just BCIP for detection (light blue), and the status of differentiated ciliated cells was measured by staining with the 6-11B1 antibody using HRP immunohistochemistry (brown). Note that BCIP detection gives rise to a higher level of background (diffuse sky blue staining) compared to NBT-BCIP detection. (D-H) Example of an embryo with a relatively mild increase in the number of α-tubulin-expressing cells. Lateral views are shown with anterior to the left. (F,G) Magnified views of the area framed in D and E, respectively, showing that all the α-tubulin-expressing cells have differentiated since they are also stained with 6-11B1. The density of the differentiated ciliated cells approximately doubled on the injected side without affecting the morphology of the skin. (H) Transverse section through the embryo shown in D and E showing that the extra α-tubulin-expressing cells have reached the surface, resulting in a spacing pattern twice as dense on the injected side: seventeen ciliated cells can be counted on the injected side, versus nine on the uninjected side. nc, notochord; sm, somitic mesoderm; lm, lateral mesoderm; epi, epiderm. (I-N) An embryo with a severe overproduction of α- tubulin-expressing cells. (I,J,L,M) Lateral views are shown anterior to the left. (L,M) Magnified views of the area framed in I and J. Note the continuous pattern of α-tubulin staining on the injected side (compare I to J and L to M). The arrow points to a region where 6-11B1 Ab staining can be distinguished despite the high level of blue staining due to α-tubulin overexpression. (K,N) Sections through the embryo shown in I and J allow the detection of the 6-11B1 staining much better than the whole-mount view. (K) The injected area, marked by purple-stained nuclei, lies between the two arrowheads. Note that overproduction of α-tubulin-expressing cells in this area leads to a thickening and altered morphology of the epidermis. (N) A high-power view of an adjacent section shows that most of the α-tubulin-expressing cells have differentiated since they are also stained by 6-11B1 antibody whichever position they have in the ectoderm. The differentiated ciliated cells (blue and brown) that have reached the surface of the skin are surrounded by non-ciliated cells (asterisks). A fraction of the double-labeled cells were retained in the inner layer of the epidermis (arrowheads). Note that some of them are likely to be migrating to the surface (arrows) indicating that intercalation is an ongoing process. ectoderm does not activate Notch signaling as it does in the neural plate where it suppresses neuronal differentiation (Chitnis et al., 1995) but rather prevents it. Consistently, in Drosophila, much evidence has accumulated recently to support the idea that high levels of Notch ligands within a cell may make that cell unresponsive to Notch signaling (Jacobsen et al., 1998, and references therein). Another possibility would be that overactivating Notch through its ligand before the time of the actual cell fate decision would produce a high level of signaling, eventually leading to a desensitization in the responding cells at a time when the Notch pathway is normally required to control differentiation. Outer ectodermal cells appear not to give rise to α-tubulinexpressing cells even when Notch signaling is blocked (data not shown) and the reason why this layer does not produce ciliated-cell precursors will be of interest in the future. In contrast, all cells in the inner layer of the non-neural ectoderm appear to be capable of differentiating as α-tubulin-expressing cells (Fig. 8C), suggesting that they all express a factor that drives ciliated-cell differentiation. The distribution of this unknown factor would be initially relatively uniform within the inner cell layer of the ectoderm and the outcome of Notch signaling would be to silence this instructive factor in the subset of cells that are not allowed to differentiate. In an example that is comparable to the ciliated cells selection, the different cell types of the Drosophila endoderm segregate from
11 Formation of the ciliated cells spacing pattern 4725 a equipotential field which is the whole endodermal germ layer (Tepass and Hartenstein, 1995). The bhlh proneural genes were shown to be expressed homogenously throughout the endoderm, to be required for determination of cell fates and to interact with the neurogenic genes in the same way as they do in the neural ectoderm. Thus, it seems likely that cells forming the ciliated-cell precursors are selected by a feedback loop that would operate through an upstream factor, a proneural-like factor expressed by the whole inner layer, driving X-Delta-1 expression and instructing the ciliated-cell fate. Further understanding of how ciliated-cell precursors are selected depends largely on identifying this upstream factor. ESR-6e is a Notch target gene in the non-neural ectoderm We have identified a novel Xenopus WPRW-bHLH gene, called ESR-6e, which encodes a protein with all the conserved features shared by the Hairy-E(spl)-related proteins including Fig. 9. Schematic representation of the two-step mechanism spacing the ciliated cells. (1) Lateral inhibition takes place in the inner ectodermal layer at late gastrula stages and drives a subset of cells to express higher levels of X-Delta-1 (green cells). These cells inhibit the differentiation of their neighbours (orange cells) and take on the ciliated-cell fate as marked by expression of α-tubulin (blue cells). (2) At neurula stages, the inner layer cells organize into a monolayer and the α-tubulin-expressing cells intercalate into epithelial junctions of the outer layer (columnar red cells). Differentiation of the ciliated cells occurs at early tadpole stages while they reach their definitive position in the epithelium. At the same time, division of the surface layer cells produces new interstitial locations available for intercalation of additional ciliated cells. a basic Helix-Loop-Helix region that has 70% homology with ESR-1 bhlh region, a conserved proline residue in the basic domain and the C-terminal WRPW tetrapeptide, which binds the transcriptional repressor Groucho (Fisher and Caudy, 1998; Fisher et al., 1996). ESR-6e is primarily expressed within the non-neural ectoderm where it appears to have two aspects to its expression pattern. ESR-6e is detected at relatively high and uniform levels in the outer layer of the non-neural ectoderm after gastrulation is complete. In addition, ESR-6e is also detected at low levels in the inner layer of ectoderm during gastrulation when ciliated-cells precursors form and when X- Delta-1 is expressed. This latter aspect of ESR-6e expression is consistent with a role as a Notch target gene during the selection of ciliated-cell precursors. Indeed expression of ESR- 6e in both layers can be upregulated by an activated form of Notch, indicating that Notch signaling is one input promoting the expression of ESR-6e. Notably, ESR-6e expression cannot be induced in the neural plate by Notch-ICD, suggesting that Notch signaling is not sufficient for ESR-6e expression in all regions of the ectoderm. These results parallel those obtained for other WRPW-bHLH genes that are targets of Notch signaling in Xenopus in that, in most cases, these genes are only expressed in a particular tissue when Notch signaling is active. For example, ESR-1 and ESR-7 are activated by Notch signaling in the neural plate during primary neurogenesis, while ESR-4 and ESR-5 are activated by Notch signaling in the paraxial mesoderm during segmentation (Jen et al., 1999). Because these different Notch target genes have tissue specificity, other factors likely modulate their ability to respond to Notch signaling. In the case of ESR-6e, it will be interesting to know why Notch-ICD preferentially induces its expression in the non-neural ectoderm and how this preference relates to the role of the BMP pathway in directing ectoderm into a nonneural fate. The expression of ESR-6e in the inner cell layer occurs at a time when X-Delta-1 is expressed, prefiguring the appearance of ciliated-cell precursors. In addition, expression of ESR-6e can be induced in the inner cell layer by Notch-ICD, suggesting that it is a Notch target gene. However, X-Su(H)-DBM, an inhibitor of Notch signaling, activates the expression of ESR- 6e in the inner layer. On one hand, this result was unanticipated for a target of a Su(H)-mediated Notch signaling, since X- Su(H)-DBM blocks the expression of other Xenopus ESR genes such as ESR-1, ESR-4, ESR-5 and ESR-7 (Jen et al., 1999; Wettstein et al., 1997) and not shown). On the other hand, this result is fully consistent with observations made on the regulation of the E(spl)-C genes in Drosophila, which were shown to be direct targets of both the Notch-activated Su(H) and the proneural bhlh proteins Achaete and Scute (Bailey and Posakony, 1995; Lecourtois and Schweisguth, 1995; Singson et al., 1994). Like ESR-6e, the expression of these genes increases when Notch signaling is blocked, presumably because loss of Notch function leads to higher levels of ac-sc expression, which in turn drive higher expression levels of the E(spl) genes via E-boxes present in their promoters. From these observations and the extensive analysis of the E(spl)-C gene promoters, Posakony and colleagues have proposed that dual transcriptional activation by Su(H) and bhlh proteins is the rule for eight out of nine genes in the E(spl) complex (Nellesen et al., 1999) and that this dual regulation is conserved amongst divergent Drosophila species. By analogy, we propose that the
Neural Induction. Chapter One
 Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
Neural Induction Chapter One Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Mesodermal Induction Lateral Inhibition
7.22 Final Exam points
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
+ + Development and Evolution Dorsoventral axis. Developmental Readout. Foundations. Stem cells. Organ formation.
 Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
7.22 Example Problems for Exam 1 The exam will be of this format. It will consist of 2-3 sets scenarios.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Chapter 14 Regulation of Transcription
 Chapter 14 Regulation of Transcription Cis-acting sequences Distance-independent cis-acting elements Dissecting regulatory elements Transcription factors Overview transcriptional regulation Transcription
Chapter 14 Regulation of Transcription Cis-acting sequences Distance-independent cis-acting elements Dissecting regulatory elements Transcription factors Overview transcriptional regulation Transcription
Mesoderm Formation. Fate map of early gastrula. Only two types of mesoderm are induced. Mesoderm induction by the vegetal hemisphere
 Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Xenopus gastrulation. Dorsal-Ventral Patterning - The Spemann Organizer. Two mesoderm inducing signals
 Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Supporting Information
 Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Readings. Lecture IV. Mechanisms of Neural. Neural Development. September 10, Bio 3411 Lecture IV. Mechanisms of Neural Development
 Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Fertilization. Animal hemisphere. Sperm entry point
 Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Gene Expression: Transcription
 Gene Expression: Transcription The majority of genes are expressed as the proteins they encode. The process occurs in two steps: Transcription = DNA RNA Translation = RNA protein Taken together, they make
Gene Expression: Transcription The majority of genes are expressed as the proteins they encode. The process occurs in two steps: Transcription = DNA RNA Translation = RNA protein Taken together, they make
Lecture 3 MOLECULAR REGULATION OF DEVELOPMENT
 Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Neural Development. How does a single cell make a brain??? How are different brain regions specified??? Neural Development
 Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and
 Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
sides of the aleurone (Al) but it is excluded from the basal endosperm transfer layer
 Supplemental Data. Gómez et al. (2009). The maize transcription factor MRP-1 (Myb-Related-Protein-1) is a key regulator of the differentiation of transfer cells. Supplemental Figure 1. Expression analyses
Supplemental Data. Gómez et al. (2009). The maize transcription factor MRP-1 (Myb-Related-Protein-1) is a key regulator of the differentiation of transfer cells. Supplemental Figure 1. Expression analyses
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development
 Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Supporting Information
 Supporting Information Ho et al. 10.1073/pnas.0808899106 SI Materials and Methods In immunostaining, antibodies from Developmental Studies Hybridoma ank were -FasII (mouse, 1:200), - (mouse, 1:100), and
Supporting Information Ho et al. 10.1073/pnas.0808899106 SI Materials and Methods In immunostaining, antibodies from Developmental Studies Hybridoma ank were -FasII (mouse, 1:200), - (mouse, 1:100), and
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
Early Development and Axis Formation in Amphibians
 Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Supplemental Figure 1 (Figure S1), related to Figure 1 Figure S1 provides evidence to demonstrate Nfatc1Cre is a mouse line that directed gene
 Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
7.012 Problem Set 5. Question 1
 Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Name Section 7.012 Problem Set 5 Question 1 While studying the problem of infertility, you attempt to isolate a hypothetical rabbit gene that accounts for the prolific reproduction of rabbits. After much
Pattern Formation via Small RNA Mobility SUPPLEMENTAL FIGURE 1 SUPPLEMENTAL INFORMATION. Daniel H. Chitwood et al.
 SUPPLEMENTAL INFORMATION Pattern Formation via Small RNA Mobility Daniel H. Chitwood et al. SUPPLEMENTAL FIGURE 1 Supplemental Figure 1. The ARF3 promoter drives expression throughout leaves. (A, B) Expression
SUPPLEMENTAL INFORMATION Pattern Formation via Small RNA Mobility Daniel H. Chitwood et al. SUPPLEMENTAL FIGURE 1 Supplemental Figure 1. The ARF3 promoter drives expression throughout leaves. (A, B) Expression
TRANSGENIC ANIMALS. transient. stable. - Two methods to produce transgenic animals:
 Only for teaching purposes - not for reproduction or sale CELL TRANSFECTION transient stable TRANSGENIC ANIMALS - Two methods to produce transgenic animals: 1- DNA microinjection 2- embryonic stem cell-mediated
Only for teaching purposes - not for reproduction or sale CELL TRANSFECTION transient stable TRANSGENIC ANIMALS - Two methods to produce transgenic animals: 1- DNA microinjection 2- embryonic stem cell-mediated
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Schematic representation of the endogenous PALB2 locus and gene-disruption constructs
 Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Later Development. Caenorhabditis elegans. Later Processes 10/06/12. Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development
 Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Concepts and Methods in Developmental Biology
 Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Lecture 20: Drosophila melanogaster
 Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene. Andrew ElBardissi, The Pennsylvania State University
 Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene Andrew ElBardissi, The Pennsylvania State University Abstract: Hong Ma, The Pennsylvania State University The Excess Microsporocytes
Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene Andrew ElBardissi, The Pennsylvania State University Abstract: Hong Ma, The Pennsylvania State University The Excess Microsporocytes
RNAs were transcribed from described expression conhibit a similar capacity to synergize with neural-inducing
 DEVELOPMENTAL BIOLOGY 172, 337 342 (1995) RAPID COMMUNICATION Specification of the Anteroposterior Neural Axis through Synergistic Interaction of the Wnt Signaling Cascade with noggin and follistatin L.
DEVELOPMENTAL BIOLOGY 172, 337 342 (1995) RAPID COMMUNICATION Specification of the Anteroposterior Neural Axis through Synergistic Interaction of the Wnt Signaling Cascade with noggin and follistatin L.
Roche Molecular Biochemicals Technical Note No. LC 12/2000
 Roche Molecular Biochemicals Technical Note No. LC 12/2000 LightCycler Absolute Quantification with External Standards and an Internal Control 1. General Introduction Purpose of this Note Overview of Method
Roche Molecular Biochemicals Technical Note No. LC 12/2000 LightCycler Absolute Quantification with External Standards and an Internal Control 1. General Introduction Purpose of this Note Overview of Method
Exam 1 ID#: October 1, 2006
 Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Somatic Primary pirna Biogenesis Driven by cis-acting RNA Elements and Trans-Acting Yb
 Cell Reports Supplemental Information Somatic Primary pirna Biogenesis Driven by cis-acting RNA Elements and Trans-Acting Yb Hirotsugu Ishizu, Yuka W. Iwasaki, Shigeki Hirakata, Haruka Ozaki, Wataru Iwasaki,
Cell Reports Supplemental Information Somatic Primary pirna Biogenesis Driven by cis-acting RNA Elements and Trans-Acting Yb Hirotsugu Ishizu, Yuka W. Iwasaki, Shigeki Hirakata, Haruka Ozaki, Wataru Iwasaki,
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
Supplemental Information
 Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Lecture 20: Drosophila embryogenesis
 Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
Neural Induction. Steven McLoon Department of Neuroscience University of Minnesota
 Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
Neural Induction Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour (with Dr. Nakagawa) Friday, Sept 22 8:30-9:30am Surdyks Café in Northrop Auditorium Stop by for
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
Supplemental Data. Na Xu et al. (2016). Plant Cell /tpc
 Supplemental Figure 1. The weak fluorescence phenotype is not caused by the mutation in At3g60240. (A) A mutation mapped to the gene At3g60240. Map-based cloning strategy was used to map the mutated site
Supplemental Figure 1. The weak fluorescence phenotype is not caused by the mutation in At3g60240. (A) A mutation mapped to the gene At3g60240. Map-based cloning strategy was used to map the mutated site
Technical Review. Real time PCR
 Technical Review Real time PCR Normal PCR: Analyze with agarose gel Normal PCR vs Real time PCR Real-time PCR, also known as quantitative PCR (qpcr) or kinetic PCR Key feature: Used to amplify and simultaneously
Technical Review Real time PCR Normal PCR: Analyze with agarose gel Normal PCR vs Real time PCR Real-time PCR, also known as quantitative PCR (qpcr) or kinetic PCR Key feature: Used to amplify and simultaneously
Roche Molecular Biochemicals Technical Note No. LC 10/2000
 Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
Name AP Biology Mrs. Laux Take home test #11 on Chapters 14, 15, and 17 DUE: MONDAY, DECEMBER 21, 2009
 MULTIPLE CHOICE QUESTIONS 1. Inducible genes are usually actively transcribed when: A. the molecule degraded by the enzyme(s) is present in the cell. B. repressor molecules bind to the promoter. C. lactose
MULTIPLE CHOICE QUESTIONS 1. Inducible genes are usually actively transcribed when: A. the molecule degraded by the enzyme(s) is present in the cell. B. repressor molecules bind to the promoter. C. lactose
Neural crest induction in Xenopus: evidence for a two-signal model
 Development 125, 2403-2414 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV4980 2403 Neural crest induction in Xenopus: evidence for a two-signal model Carole LaBonne* and Marianne
Development 125, 2403-2414 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV4980 2403 Neural crest induction in Xenopus: evidence for a two-signal model Carole LaBonne* and Marianne
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
CHAPTER 9 DNA Technologies
 CHAPTER 9 DNA Technologies Recombinant DNA Artificially created DNA that combines sequences that do not occur together in the nature Basis of much of the modern molecular biology Molecular cloning of genes
CHAPTER 9 DNA Technologies Recombinant DNA Artificially created DNA that combines sequences that do not occur together in the nature Basis of much of the modern molecular biology Molecular cloning of genes
TRANSGENIC ANIMALS. -transient transfection of cells -stable transfection of cells. - Two methods to produce transgenic animals:
 TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
Xenopus embryos. The role of F-cadherin in localizing cells during neural tube formation in
 Development 125, 1-312 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV1196 1 The role of F-cadherin in localizing cells during neural tube formation in Xenopus embryos Amy Espeseth
Development 125, 1-312 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV1196 1 The role of F-cadherin in localizing cells during neural tube formation in Xenopus embryos Amy Espeseth
Molecular Cell Biology - Problem Drill 11: Recombinant DNA
 Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10810 Supplementary Fig. 1: Mutation of the loqs gene leads to shortened lifespan and adult-onset brain degeneration. a. Northern blot of control and loqs f00791 mutant flies. loqs f00791
doi:10.1038/nature10810 Supplementary Fig. 1: Mutation of the loqs gene leads to shortened lifespan and adult-onset brain degeneration. a. Northern blot of control and loqs f00791 mutant flies. loqs f00791
Figure S1 Correlation in size of analogous introns in mouse and teleost Piccolo genes. Mouse intron size was plotted against teleost intron size for t
 Figure S1 Correlation in size of analogous introns in mouse and teleost Piccolo genes. Mouse intron size was plotted against teleost intron size for the pcloa genes of zebrafish, green spotted puffer (listed
Figure S1 Correlation in size of analogous introns in mouse and teleost Piccolo genes. Mouse intron size was plotted against teleost intron size for the pcloa genes of zebrafish, green spotted puffer (listed
a) Stock 4: 252 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males.
![a) Stock 4: 252 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males. a) Stock 4: 252 flies total: 120 are P[w+]: 64 are CyO; 56 are TM3; all are females. 132 are w-: 68 are CyO; 64 are TM3; all are males.](/thumbs/91/106088469.jpg) Drosophila Problem Set 2018 1) You have created P element transformants of a construct that contains the mini-white gene, which confers an orange eye color in a homozygous white mutant background. For
Drosophila Problem Set 2018 1) You have created P element transformants of a construct that contains the mini-white gene, which confers an orange eye color in a homozygous white mutant background. For
CAP BIOINFORMATICS Su-Shing Chen CISE. 10/5/2005 Su-Shing Chen, CISE 1
 CAP 5510-9 BIOINFORMATICS Su-Shing Chen CISE 10/5/2005 Su-Shing Chen, CISE 1 Basic BioTech Processes Hybridization PCR Southern blotting (spot or stain) 10/5/2005 Su-Shing Chen, CISE 2 10/5/2005 Su-Shing
CAP 5510-9 BIOINFORMATICS Su-Shing Chen CISE 10/5/2005 Su-Shing Chen, CISE 1 Basic BioTech Processes Hybridization PCR Southern blotting (spot or stain) 10/5/2005 Su-Shing Chen, CISE 2 10/5/2005 Su-Shing
Chromosomal Mutations. 2. Gene Mutations
 12-4 12-4 1. Chromosomal 3. NOT! 2. Gene A genetic mutation is any change in the DNA nucleotide sequence. Mutation is caused by mistakes during DNA replication, as well as mutagens, like certain chemicals
12-4 12-4 1. Chromosomal 3. NOT! 2. Gene A genetic mutation is any change in the DNA nucleotide sequence. Mutation is caused by mistakes during DNA replication, as well as mutagens, like certain chemicals
DNA Microarray Technology
 CHAPTER 1 DNA Microarray Technology All living organisms are composed of cells. As a functional unit, each cell can make copies of itself, and this process depends on a proper replication of the genetic
CHAPTER 1 DNA Microarray Technology All living organisms are composed of cells. As a functional unit, each cell can make copies of itself, and this process depends on a proper replication of the genetic
Learning Objectives :
 Learning Objectives : Understand the basic differences between genomic and cdna libraries Understand how genomic libraries are constructed Understand the purpose for having overlapping DNA fragments in
Learning Objectives : Understand the basic differences between genomic and cdna libraries Understand how genomic libraries are constructed Understand the purpose for having overlapping DNA fragments in
7.06 Cell Biology EXAM #2 March 20, 2003
 7.06 Cell Biology EXAM #2 March 20, 2003 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #2 March 20, 2003 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
Site directed mutagenesis, Insertional and Deletion Mutagenesis. Mitesh Shrestha
 Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
SUPPLEMENTARY INFORMATION
 Ca 2+ /calmodulin Regulates Salicylic Acid-mediated Plant Immunity Liqun Du, Gul S. Ali, Kayla A. Simons, Jingguo Hou, Tianbao Yang, A.S.N. Reddy and B. W. Poovaiah * *To whom correspondence should be
Ca 2+ /calmodulin Regulates Salicylic Acid-mediated Plant Immunity Liqun Du, Gul S. Ali, Kayla A. Simons, Jingguo Hou, Tianbao Yang, A.S.N. Reddy and B. W. Poovaiah * *To whom correspondence should be
Problem Set 8. Answer Key
 MCB 102 University of California, Berkeley August 11, 2009 Isabelle Philipp Online Document Problem Set 8 Answer Key 1. The Genetic Code (a) Are all amino acids encoded by the same number of codons? no
MCB 102 University of California, Berkeley August 11, 2009 Isabelle Philipp Online Document Problem Set 8 Answer Key 1. The Genetic Code (a) Are all amino acids encoded by the same number of codons? no
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
Selected Techniques Part I
 1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
Biology 201 (Genetics) Exam #3 120 points 20 November Read the question carefully before answering. Think before you write.
 Name KEY Section Biology 201 (Genetics) Exam #3 120 points 20 November 2006 Read the question carefully before answering. Think before you write. You will have up to 50 minutes to take this exam. After
Name KEY Section Biology 201 (Genetics) Exam #3 120 points 20 November 2006 Read the question carefully before answering. Think before you write. You will have up to 50 minutes to take this exam. After
7.013 Practice Quiz
 MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
MIT Department of Biology 7.013: Introductory Biology - Spring 2005 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Practice Quiz 2 2004 1 Question 1 A. The primer
Design. Construction. Characterization
 Design Construction Characterization DNA mrna (messenger) A C C transcription translation C A C protein His A T G C T A C G Plasmids replicon copy number incompatibility selection marker origin of replication
Design Construction Characterization DNA mrna (messenger) A C C transcription translation C A C protein His A T G C T A C G Plasmids replicon copy number incompatibility selection marker origin of replication
Chapter 20 Biotechnology
 Chapter 20 Biotechnology Manipulation of DNA In 2007, the first entire human genome had been sequenced. The ability to sequence an organisms genomes were made possible by advances in biotechnology, (the
Chapter 20 Biotechnology Manipulation of DNA In 2007, the first entire human genome had been sequenced. The ability to sequence an organisms genomes were made possible by advances in biotechnology, (the
OriGene GFC-Arrays for High-throughput Overexpression Screening of Human Gene Phenotypes
 OriGene GFC-Arrays for High-throughput Overexpression Screening of Human Gene Phenotypes High-throughput Gene Function Validation Tool Introduction sirna screening libraries enable scientists to identify
OriGene GFC-Arrays for High-throughput Overexpression Screening of Human Gene Phenotypes High-throughput Gene Function Validation Tool Introduction sirna screening libraries enable scientists to identify
PLNT2530 (2018) Unit 6b Sequence Libraries
 PLNT2530 (2018) Unit 6b Sequence Libraries Molecular Biotechnology (Ch 4) Analysis of Genes and Genomes (Ch 5) Unless otherwise cited or referenced, all content of this presenataion is licensed under the
PLNT2530 (2018) Unit 6b Sequence Libraries Molecular Biotechnology (Ch 4) Analysis of Genes and Genomes (Ch 5) Unless otherwise cited or referenced, all content of this presenataion is licensed under the
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
Yeast 2-Hybrid Kayla Nygaard
 Yeast 2-Hybrid 2.26.18 Kayla Nygaard Y2H - What is it? A method to screen for protein-protein interactions in yeast Capitalizes on GAL4 system in yeast. GAL4 has 2 domains DNA-Binding Domain (DB) Transcriptional
Yeast 2-Hybrid 2.26.18 Kayla Nygaard Y2H - What is it? A method to screen for protein-protein interactions in yeast Capitalizes on GAL4 system in yeast. GAL4 has 2 domains DNA-Binding Domain (DB) Transcriptional
Characterization of a novel gene involved in border cell migration
 Characterization of a novel gene involved in border cell migration Wei-Hao Li, He-Yen Chou and Li-Mei Pai Graduate Institute of Basic Medical Science Chang-Gung University, Tao-Yuan, Taiwan, R.O.C. Abstract
Characterization of a novel gene involved in border cell migration Wei-Hao Li, He-Yen Chou and Li-Mei Pai Graduate Institute of Basic Medical Science Chang-Gung University, Tao-Yuan, Taiwan, R.O.C. Abstract
Chapter 6 - Molecular Genetic Techniques
 Chapter 6 - Molecular Genetic Techniques Two objects of molecular & genetic technologies For analysis For generation Molecular genetic technologies! For analysis DNA gel electrophoresis Southern blotting
Chapter 6 - Molecular Genetic Techniques Two objects of molecular & genetic technologies For analysis For generation Molecular genetic technologies! For analysis DNA gel electrophoresis Southern blotting
CHAPTER 20 DNA TECHNOLOGY AND GENOMICS. Section A: DNA Cloning
 Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Section A: DNA Cloning 1. DNA technology makes it possible to clone genes for basic research and commercial applications: an overview 2. Restriction enzymes are used to make recombinant DNA 3. Genes can
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Figure S2. Verification of ihog and boi
 Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
Biotechnology. Chapter 20. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 20 Biotechnology PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Chapter 20 Biotechnology PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Bootcamp: Molecular Biology Techniques and Interpretation
 Bootcamp: Molecular Biology Techniques and Interpretation Bi8 Winter 2016 Today s outline Detecting and quantifying nucleic acids and proteins: Basic nucleic acid properties Hybridization PCR and Designing
Bootcamp: Molecular Biology Techniques and Interpretation Bi8 Winter 2016 Today s outline Detecting and quantifying nucleic acids and proteins: Basic nucleic acid properties Hybridization PCR and Designing
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms
 Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
Gene Expression Technology
 Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling
 Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Developmental Biology 3230 Exam 1 (Feb. 6) NAME
 DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
Supplementary Information
 Supplementary Information Supplementary Figure 1: Xenopus model of missense mutations. A mutation equivalent to MCIDAS R381H/G355D was introduced in the Xenopus mcidas (R370H, G355D) by PCR, sequenced
Supplementary Information Supplementary Figure 1: Xenopus model of missense mutations. A mutation equivalent to MCIDAS R381H/G355D was introduced in the Xenopus mcidas (R370H, G355D) by PCR, sequenced
ANAT2341 Embryology Introduction: Steve Palmer
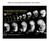 ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
Cloning, chromosome localization and features of a novel human gene, MATH2
 Indian Academy of Sciences Cloning, chromosome localization and features of a novel human gene, MATH2 LINGCHEN GUO 1, MIN JIANG 1, YUSHU MA 1, HAIPENG CHENG 1, XIAOHUA NI 1, YANGSHENG JIN 2, YI XIE 1 *
Indian Academy of Sciences Cloning, chromosome localization and features of a novel human gene, MATH2 LINGCHEN GUO 1, MIN JIANG 1, YUSHU MA 1, HAIPENG CHENG 1, XIAOHUA NI 1, YANGSHENG JIN 2, YI XIE 1 *
Quantitative Real time PCR. Only for teaching purposes - not for reproduction or sale
 Quantitative Real time PCR PCR reaction conventional versus real time PCR real time PCR principles threshold cycle C T efficiency relative quantification reference genes primers detection chemistry GLP
Quantitative Real time PCR PCR reaction conventional versus real time PCR real time PCR principles threshold cycle C T efficiency relative quantification reference genes primers detection chemistry GLP
Supplementary Figure 1. Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings.
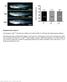 Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Molecular Genetics Techniques. BIT 220 Chapter 20
 Molecular Genetics Techniques BIT 220 Chapter 20 What is Cloning? Recombinant DNA technologies 1. Producing Recombinant DNA molecule Incorporate gene of interest into plasmid (cloning vector) 2. Recombinant
Molecular Genetics Techniques BIT 220 Chapter 20 What is Cloning? Recombinant DNA technologies 1. Producing Recombinant DNA molecule Incorporate gene of interest into plasmid (cloning vector) 2. Recombinant
Recent technology allow production of microarrays composed of 70-mers (essentially a hybrid of the two techniques)
 Microarrays and Transcript Profiling Gene expression patterns are traditionally studied using Northern blots (DNA-RNA hybridization assays). This approach involves separation of total or polya + RNA on
Microarrays and Transcript Profiling Gene expression patterns are traditionally studied using Northern blots (DNA-RNA hybridization assays). This approach involves separation of total or polya + RNA on
Student name ID # Second Mid Term Exam, Biology 2020, Spring 2002 Scores Total
 Second Mid Term Exam, Biology 2020, Spring 2002 Scores 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. Total 1 1. Matching (7 pts). Each answer is used exactly once Helicase
Second Mid Term Exam, Biology 2020, Spring 2002 Scores 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. Total 1 1. Matching (7 pts). Each answer is used exactly once Helicase
Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mot
 Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mottershead* Open Access Address: Programme for Developmental
Reproductive Biology and Endocrinology BioMed Central Review Smad signalling in the ovary Noora Kaivo-oja, Luke A Jeffery, Olli Ritvos and David G Mottershead* Open Access Address: Programme for Developmental
Lecture 8: Transgenic Model Systems and RNAi
 Lecture 8: Transgenic Model Systems and RNAi I. Model systems 1. Caenorhabditis elegans Caenorhabditis elegans is a microscopic (~1 mm) nematode (roundworm) that normally lives in soil. It has become one
Lecture 8: Transgenic Model Systems and RNAi I. Model systems 1. Caenorhabditis elegans Caenorhabditis elegans is a microscopic (~1 mm) nematode (roundworm) that normally lives in soil. It has become one
(phosphatase tensin) domain is shown in dark gray, the FH1 domain in black, and the
 Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15
 Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
LIFE Formation III. Morphogenesis: building 3D structures START FUTURE. How-to 2 PROBLEMS FORMATION SYSTEMS SYSTEMS.
 7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
What s the most complex problem in biology?
 Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Enhancers mutations that make the original mutant phenotype more extreme. Suppressors mutations that make the original mutant phenotype less extreme
 Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
Applicazioni biotecnologiche
 Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
