Lef1 regulates Dusp6 to influence neuromast formation and spacing in the zebrafish posterior lateral line primordium
|
|
|
- Sabina Burns
- 6 years ago
- Views:
Transcription
1 RESEARCH ARTICLE 2387 Development 140, (2013) doi: /dev Published by The Company of Biologists Ltd Lef1 regulates Dusp6 to influence neuromast formation and spacing in the zebrafish posterior lateral line primordium Miho Matsuda 1,2, Damian Dalle Nogare 1, Katherine Somers 1, Kathleen Martin 1, Chongmin Wang 1 and Ajay B. Chitnis 1, * SUMMARY The posterior lateral line primordium (PLLp) migrates caudally and periodically deposits neuromasts. Coupled, but mutually inhibitory, Wnt-FGF signaling systems regulate proto-neuromast formation in the PLLp: FGF ligands expressed in response to Wnt signaling activate FGF receptors and initiate proto-neuromast formation. FGF receptor signaling, in turn, inhibits Wnt signaling. However, mechanisms that determine periodic neuromast formation and deposition in the PLLp remain poorly understood. Previous studies showed that neuromasts are deposited closer together and the PLLp terminates prematurely in lef1-deficient zebrafish embryos. It was suggested that this results from reduced proliferation in the leading domain of the PLLp and/or premature incorporation of progenitors into proto-neuromasts. We found that rspo3 knockdown reduces proliferation in a manner similar to that seen in lef1 morphants. However, it does not cause closer neuromast deposition or premature termination of the PLLp, suggesting that such changes in lef1-deficient embryos are not linked to changes in proliferation. Instead, we suggest that they are related to the role of Lef1 in regulating the balance of Wnt and FGF functions in the PLLp. Lef1 determines expression of the FGF signaling inhibitor Dusp6 in leading cells and regulates incorporation of cells into neuromasts; reduction of Dusp6 in leading cells in lef1-deficient embryos allows new proto-neuromasts to form closer to the leading edge. This is associated with progressively slower PLLp migration, reduced spacing between deposited neuromasts and premature termination of the PLLp system. KEY WORDS: Lateral line, Wnt, FGF, Lef1, R-spondin, Dusp6, Zebrafish INTRODUCTION The lateral line in zebrafish has emerged as an attractive model system with which to understand molecular mechanisms underlying the coordination of cell proliferation, migration and differentiation during organ morphogenesis in vertebrate embryonic development (reviewed by Chitnis et al., 2012; Ma and Raible, 2009). In this study we describe a new role for Lef1 in inhibiting FGF signaling and show how the balance of Wnt and FGF signaling influences neuromast formation and deposition. The lateral line is a mechanosensory system that has evolved to detect patterns of water movement (Coombs and van Netten, 2006). It contains neuromasts, which are small sensory organs that are distributed in a stereotyped pattern on the surface of the fish. Formation of the posterior lateral line system in zebrafish is pioneered by the posterior lateral line primordium (PLLp), a cluster of ~100 cells formed near the ear that migrates under the skin along the horizontal myoseptum and periodically deposits five to six neuromasts during its migration (reviewed by Ghysen and Dambly- Chaudière, 2007). The PLLp eventually dissociates to form two to three terminal neuromasts at the tip of the tail. Formation of nascent neuromasts, or proto-neuromasts, is initiated by FGF signaling in the migrating PLLp (Lecaudey et al., 2008; Nechiporuk and Raible, 2008). Fgf3 and Fgf10 are expressed in response to Wnt signaling in a leading domain of the PLLp 1 Program in Genomics of Development, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20852, USA. 2 Department of Cell Biology and Molecular Medicine, University of Medicine and Dentistry of New Jersey, New Jersey Medical School, Newark, NJ 07103, USA. *Author for correspondence (chitnisa@mail.nih.gov) Accepted 3 April 2013 (Aman and Piotrowski, 2008). However, the FGFs are not very effective at activating FGF receptor (FGFR) signaling in the leading domain, at least in part because factors that determine FGF expression also determine the expression of Sef (Il17rd Zebrafish Information Network), an inhibitor of FGFR signaling (Aman and Piotrowski, 2008; Tsang et al., 2002). As a result, FGFs produced in the leading domain in response to Wnt signaling activate FGFR signaling in an adjacent trailing domain, where there is less inhibition of FGFR signaling. FGFR signaling also inhibits Wnt signaling and restricts expression of the Wnt effector lef1 to a leading domain of the PLLp. This inhibition is mediated in part by a diffusible Wnt antagonist, Dkk1, the expression of which at the trailing end of the leading lef1 expression domain is dependent on FGFR signaling (Aman and Piotrowski, 2008). In this manner, coupled, but mutually inhibitory, Wnt-FGF signaling systems are established in the PLLp. FGF signaling induces proto-neuromast formation by initiating epithelial rosette formation and by inducing the expression of atoh1a, which gives cells the potential to become sensory hair cell progenitors (Lecaudey et al., 2008; Nechiporuk and Raible, 2008). Lateral inhibition mediated by Notch signaling restricts atoh1a expression and sensory hair cell progenitor fate to a central cell in each forming proto-neuromast (Itoh and Chitnis, 2001; Sarrazin et al., 2006). While Wnt signaling initiates fgf10 expression at the leading end of the PLLp, Atoh1a begins to drive fgf10 expression in the trailing part of the PLLp (Matsuda and Chitnis, 2010). In this manner, the central cell maintains a restricted source of FGF ligand in each maturing proto-neuromast. Proto-neuromasts are formed periodically toward the leading end of the PLLp and as they mature they are deposited from the trailing end. Chemokine signaling and the polarized expression of two chemokine receptors in the PLLp determine the directional migration of the PLLp (David et al., 2002; Haas and Gilmour, 2006;
2 2388 RESEARCH ARTICLE Development 140 (11) Dambly-Chaudière et al., 2007; Valentin et al., 2007). Whereas cells in a leading domain of the PLLp express cxcr4b and undergo collective migration, cells in the trailing part of the PLLp express cxcr7b during and after deposition (Dambly-Chaudière et al., 2007; Valentin et al., 2007). Although previous studies have examined mechanisms that initiate proto-neuromast formation and determine PLLp migration, mechanisms that regulate periodic neuromast formation and their deposition remain poorly defined. It has been suggested that cell proliferation in the PLLp is a key determinant of periodic neuromast deposition (Aman et al., 2011). These authors suggest that a regulatory mechanism keeps the cxcr7b-free leading domain relatively constant in size. In this context, the rate at which the PLLp grows by proliferation determines how quickly cells enter the trailing domain, where they express cxcr7b and are eventually deposited. This hypothesis provides a potential explanation for why manipulations that either broadly increase cell death or broadly reduce proliferation in the PLLp decrease the rate of proto-neuromast deposition in the migrating PLLp (Aman et al., 2011). Wnt and FGFR signaling both regulate proliferation in the PLLp (Aman et al., 2011). Loss of lef1, a transcriptional co-factor that mediates a subset of Wnt-dependent processes in the PLLp, reduces proliferation in the PLLp (Gamba et al., 2010; Valdivia et al., 2011; McGraw et al., 2011). However, the reduced proliferation is not accompanied by a delay in neuromast deposition. Instead, loss of lef1 function is associated with closer neuromast deposition and premature termination of the PLLp system. It has been suggested that this is because proliferation is specifically reduced in a leading domain of the PLLp when lef1 function is lost (Valdivia et al., 2011). This, the authors suggest, results in rapid shrinking of the leading domain, which is a source of hypothetical factors that determine both the length of the column of cells undergoing collective migration and how close to the leading end new proto-neuromasts form. Proliferation-related shrinking of the leading domain in lef1 mutants is suggested to accelerate the loss of these hypothetical factors, which reduces the length of the migrating column of PLLp cells, accelerates the deposition of neuromasts and eventually contributes to premature termination of the PLLp system (Valdivia et al., 2011). Alternatively, it has been suggested that the premature termination of the PLLp system is related less to reduced proliferation and more to a failure in the maintenance of progenitor cells in the leading domain of the PLLp (McGraw et al., 2011). These authors show that loss of lef1 allows premature incorporation of leading progenitors into forming proto-neuromasts, and loss of progenitors results in the premature termination of the PLLp system in lef1 mutants. However, the molecular mechanisms by which Lef1 maintains the progenitor state were not defined. We found that knockdown of rspo3, which encodes a potential facilitator of Wnt signaling (Binnerts et al., 2007; Hendrickx and Leyns, 2008), reduces proliferation in the PLLp in a manner similar to that seen following lef1 knockdown. However, it does not reduce the spacing of deposited neuromasts or result in premature termination of the PLLp system. This suggested that changes in the pattern of proliferation might not be at the heart of why neuromasts are deposited closer or why the PLLp terminates prematurely when lef1 function is lost. In this study, we have explored the possibility that these changes are more related to a distinct role of lef1 in regulating the balance of Wnt and FGF signaling and to FGF signaling-dependent formation of proto-neuromasts in the PLLp. We show that the balance of Wnt and FGF signaling can influence the neuromast deposition rate and that Lef1 determines the expression of an antagonist of FGF signaling, Dusp6, in the leading domain of the PLLp. Consistent with the role for lef1 suggested by McGraw et al. (McGraw et al., 2011), we show that Dusp6 operates downstream of lef1 to deter incorporation of cells into protoneuromasts. Taken together, our study shows how Lef1 regulates the balance of Wnt and FGF signaling and the incorporation of progenitors into nascent proto-neuromasts. MATERIALS AND METHODS Fish maintenance and mutant strains Zebrafish were maintained under standard conditions. The embryos were staged according to Kimmel et al. (Kimmel et al., 1995). The tg[cldnb:lyngfp] transgenic strain was described previously (Haas and Gilmour, 2006). Embryos were treated with 1-50 µm IWR-1 (Calbiochem), 10 µm XAV939 (Sigma), µm BCI (Calbiochem) and 20 µm SU5402 (Calbiochem) from 26 hpf to the time of fixation. Morpholino (MO) injections MOs used were (5-3 ): fgf10-mo1, GCTTTACTCACTG TACG - GATCGTCC (Nechiporuk and Raible, 2008); fgf10a-mo2, GAAAATGATGCTCACCGCCCCGTAG (Norton et al., 2005); rspo3-mo, TTGCGGCTCTTACCTTTTGTTCAAG; lef1-mo, CTCCACCT - GACAACTGCGGCATTTC (Valdivia et al., 2011). Unless stated otherwise in figure legends, 2 ng fgf10, rspo3 or lef1 MO was injected. All MO injections were performed with co-injection of 1.5 ng p53-mo (Robu et al., 2007). Standard Control Oligo (Gene Tools) was used as control-mo and injected at the same concentration as the experimental MO(s). Whole-mount in situ hybridization and immunohistochemistry Whole-mount in situ hybridization was performed as described (Matsuda and Chitnis. 2010). RNA probes were synthesized using a DIG labeling kit (Roche). BCIP/NBT substrate (Vector Laboratories) was used as a chromogen. Immunohistochemistry was performed as described (Matsuda and Chitnis, 2009). Antibodies were mouse anti-zo-1 (Invitrogen; 1:500) and rabbit anti-cldnb produced by injection of peptide based on published sequence (López-Schier et al., 2004) (1:500). Images were captured with a Zeiss LSM510. Quantification of expression domains For quantification of gene expression visualized with in situ hybridization, the length of the expression domain or its integrated density was measured. To measure integrated density, images were converted to grayscale, inverted, and the sum of the grayscale values of pixels within a region of interest (ROI) measured using the integrated density measurement plug-in in ImageJ software (NIH). For statistical analysis, an unpaired Student s t- test was performed using QuickCalcs software (GraphPad). BrdU incorporation and TUNEL staining tg[cldnb:lyngfp] embryos were dechorionated and placed in 10 mm BrdU (Roche) in a solution of egg water with 15% DMSO for 1 hour with constant agitation at 28 C. Staining was performed as described (Dalle Nogare et al., 2009), and imaging was performed on a Zeiss LSM510. Images were analyzed in ImageJ using the cell counter plug-in (Kurt De Vos, University of Sheffield, UK). PLLp were divided into thirds by length, based on the Cldnb staining, and total cells and BrdU + nuclei were counted for each segment to obtain the BrdU index. TUNEL staining was performed using the ApopTag Fluorescein Detection Kit (Millipore), according to the manufacturer s directions, except that embryos were incubated overnight in labeling solution at 37 C. TUNEL-stained tg[cldnb:lyngfp] embryos were counterstained with anti-gfp antibody (Abcam). Time-lapse For time-lapse acquisition, tg[cldnb:lyngfp] embryos were anesthetized at ~28 hpf in 600 µm Tricaine (Sigma) and embedded in 0.75% low melting point agarose (Nusieve GTG, Lonza) in glass-bottom chamber slides (Nunc). Movies were acquired for hours at 5-minute intervals using an inverted Leica SP5 confocal microscope with a 20 objective lens. Quantification of divisions and migration speed was performed in ImageJ. To calculate migration speed, the distance traveled between successive
3 Lef1 regulates Dusp6 in the PLLp RESEARCH ARTICLE 2389 Fig. 1. Expression of rspo3 and lef1 is determined by similar mechanisms but knockdown of rspo3 and of lef1 have distinct effects on the pattern of neuromast deposition. (A-I) The leading end of the PLLp and caudal end of the zebrafish embryo are to the right. (A-F) The PLLp is outlined (dotted line). (A-D) Expression of rspo3 in the PLLp at 31 hpf. (A) rspo3 is expressed in the leading domain of the wildtype (WT) PLLp. (B) Heat shock-induced expression of a dominant-repressor form of TCF (ΔΝTcf ) inhibits rspo3 expression. (C,D) Exaggeration of Wnt activity, following inhibition of FGFR signaling with SU5402 (C) or in apc mutants (D), expands rspo3 expression toward the trailing domain. (E,F) rspo3 expression in the lef1 morphant PLLp (E) and lef1 expression in the rspo3 morphant PLLp (F) at 31 hpf. (G-K) The pattern of neuromast deposition in lef1 morphants and rspo3 morphants. (G-I) Neuromast deposition in tg[cldnb:lyngfp] embryos injected with lef1-mo, rspo3- MO or control-mo (conmo) at 48 hpf. White arrows indicate individual neuromasts and yellow arrowheads indicate PLLp termination. (J,K) Average position of each deposited neuromast in lef1 or rspo3 morphants relative to the distance from the otic vesicle (ov) to the tip of the tail. (H,J) Neuromasts are spaced further apart in rspo3 morphants. See supplementary material Fig. S4 for suppression of this phenotype by rspo3 mrna co-injection. (I,K) Neuromasts are spaced closer together in lef1 morphants. Error bars indicate s.d. neuromast depositions was divided by the total time between depositions as measured from the time-lapse movie. Distribution of cells with ectopic expression of Dusp6 cdna encoding mcherry or Dusp6-T2A-mCherry red fluorescent protein was inserted downstream of the hsp70 promoter. Plasmid DNA was injected into 1- to 2-cell stage tg[cldnb:lyngfp] embryos. Embryos were heat treated at 38 C for 45 minutes at 24, 30 and 36 hpf. The distribution of red fluorescent cells in deposited neuromasts and terminal neuromasts at the tail tip was counted at 56 hpf. A total of 142 cells in eight embryos expressing mcherry and 94 cells in six embryos expressing Dusp6-mCherry were analyzed. Imaging was performed on a Leica TCS SP5 with a 40 objective lens. RESULTS rspo3 is expressed in a Wnt signaling-dependent manner in the PLLp Like lef1, which is a key mediator of Wnt signaling, rspo3 is expressed in the leading part of the PLLp (Fig. 1A). As shown previously for lef1 (Aman and Piotrowski, 2008), the expression of a dominant-repressor form of TCF, ΔΝTcf, eliminated expression of rspo3 (Fig. 1B), and inhibition of FGF signaling with SU5402 was associated with slightly expanded expression of rspo3 (Fig. 1C). However, the expansion was less than that of lef1 expression, which typically expands to fill almost the entire PLLp following SU5402 treatment (Aman and Piotrowski, 2008) (data not shown). Expanded rspo3 expression was also seen in apc mutants (Fig. 1D), associated with exaggerated Wnt signaling (Hurlstone et al., 2003) and expanded lef1 expression (Aman and Piotrowski, 2008). These observations suggest that expression of rspo3, like that of lef1, is promoted by Wnt signaling. However, knockdown of lef1, which significantly reduces GFP expression in the PLLp of Wnt reporter tg(7xtcf- Xla.Siam:GFP) ia4 transgenic fish (Valdivia et al., 2011; Moro et al., 2012) (supplementary material Fig. S2), does not reduce the expression level of rspo3 (Fig. 1E). This suggests that Wnt signaling determines rspo3 expression in a Lef1-independent manner in the PLLp. In addition, knockdown of rspo3 (see supplementary material Fig. S1 for validation of the MO) did not cause any obvious change in lef1 expression (Fig. 1F). However, it did slightly reduce fluorescence in the primordium of Wnt reporter tg(7xtcf- Xla.Siam:GFP) ia4 transgenic fish (supplementary material Fig. S2), supporting the role of Rspo3 as a facilitator of Wnt signaling. Knockdown of lef1 and of rspo3 have distinct effects on neuromast deposition As expression of lef1 and rspo3 appeared to be regulated by similar mechanisms, we compared changes in the rspo3 morphants with those in lef1 morphants to look for similarities in function. Although there was some cell death in both lef1 and rspo3 morphants, co-
4 2390 RESEARCH ARTICLE Development 140 (11) Fig. 2. Although rspo3 and lef1 morphants have similar changes in cell proliferation, the size of the lef1 expression domain is only reduced in lef1 morphants. (A,B) Knockdown of lef1 and of rspo3 reduce proliferation in a similar manner. (A) Total and regional BrdU incorporation index in the PLLp of control, lef1 morphant and rspo3 morphant embryos (see also supplementary material Table S1). (B) Total and regional cell division in the PLLp of control, lef1 morphant and rspo3 morphant embryos. ns, non-significant (P>0.05); *P<0.05, **P<0.01, ***P<0.001 (see also supplementary material Table S2). Error bars indicate s.d. (C,D) The lef1 expression domain is smaller in lef1 morphants at 31 hpf. For statistical analysis, see supplementary material Fig. S5D. (E) The progressive change in the size of the lef1 domain (red) over the course of PLLp migration. (F,G) Size of the lef1 domain (relative to its average length in control embryos at 24 hpf ) plotted against the position of the PLLp along the trunk for a collection of embryos fixed between 24 and 38 hpf (see schematic in E). Red dots indicate control-mo-injected embryos and blue dots indicate either rspo3 (F) or lef1 (G) morphant embryos. Red or blue lines indicate the associated linear regression lines for each data set. A simple linear regression model and a paired t-test confirmed that the length of the lef1 domain is significantly reduced in lef1 morphants (P<0.001). injection of p53-mo minimized p53 (Tp53)-dependent cell death in the PLLp (supplementary material Fig. S3). After confirming that p53-mo on its own does not affect PLLp development, p53-mo was co-injected with experimental or control MO to suppress p53- dependent cell death in all subsequent MO experiments. In wild-type embryos, the PLLp migrates and deposits five to six neuromasts along the horizontal myoseptum and stops migration at the tip of the tail (Fig. 1G), where the PLLp dissociates to form two to three terminal neuromasts. In rspo3 morphants neuromast spacing was increased and the PLLp system rarely (1/43) terminated prematurely (Fig. 1H,J; supplementary material Fig. S4). By contrast, as shown previously (Valdivia et al., 2011; McGraw et al., 2011), neuromasts were deposited closer to each other and the PLLp system consistently terminated prematurely in lef1 morphants (Fig. 1I,K; supplementary material Fig. S5). Co-injection of rspo3 mrna helped bring neuromast spacing and the number of deposited neuromasts closer to wild-type patterns in rspo3 morphants (supplementary material Fig. S4), supporting the specificity of rspo3-mo effects. Taken together, these studies show that knockdown of lef1 and of rspo3 have different effects on the spacing of neuromasts and on PLLp termination. Knockdown of lef1 and of rspo3 result in a similar reduction in proliferation We compared proliferation patterns in lef1 and rspo3 morphants to determine whether distinct effects on proliferation might account for the differences in their patterns of neuromast deposition. lef1 and rspo3 morphants showed similar reductions in BrdU incorporation (Fig. 2A; supplementary material Table S1); the reduction was primarily in the leading and middle domains of the PLLp, which is consistent with previous analyses of lef1 mutants (McGraw et al., 2011; Valdivia et al., 2011). As changes in cell number following cell division, not just the potential for division indicated by BrdU incorporation, are expected to determine proliferation-dependent changes in neuromast deposition, time-lapse imaging of the PLLp was used to record every cell division during ~15 hours of PLLp migration, starting from just before L1 deposition (supplementary material Movies 1-3). Although there was no significant difference in the pattern of cell divisions between lef1 and rspo3 morphants, the analysis confirmed that the number of cell divisions in both lef1 and rspo3 morphants was significantly lower than in controls (Fig. 2B; supplementary material Table S2). It is important to note that, although BrdU incorporation was most prominent in the leading and middle thirds of the wild-type PLLp (Fig. 2A; supplementary material Table S1), cell divisions were relatively infrequent in the leading third and were most frequent in the middle third (Fig. 2B; supplementary material Table S2). Furthermore, the reduction in cell division was least in the leading third of the PLLp in both lef1 and rspo3 morphants (Fig. 2B). Changes in the pattern of proliferation were similar in lef1 and rspo3 morphants, but effects on neuromast deposition and PLLp
5 Lef1 regulates Dusp6 in the PLLp RESEARCH ARTICLE 2391 Fig. 3. Correlation of PLLp migration speed with spacing of deposited neuromasts. (A) PLLp migration speed compared in control, lef1 and rspo3 morphant embryos in successive periods between neuromast deposition. Red asterisk indicates that the speed between L5-L6 depositions was significantly slower than between preceding L4-L5 depositions. Blue asterisks indicate that PLLp speed in lef1 morphants between L3-L4 and L4-L5 depositions was significantly slower than in the respective, preceding deposition cycles. In addition, PLLp speed in lef1 morphants between L3-L4 and L4-L5 depositions was significantly slower than in controls during the same deposition cycle. Green asterisks indicate statistical significance between the indicated measurements (black bar). *P<0.05. Error bars indicate s.d. (B-D) PLLp migration speed plotted against distance traveled between neuromast depositions in wild-type (B), rspo3 morphant (C) and lef1 morphant (D) embryos. Points are shown in different colors for successive pairs of neuromasts (color key in D). Linear regression lines are shown for each data set. The associated coefficient of determination (R 2 ) indicates that the correlation between PLLp migration speed and distance traveled between neuromast depositions is strong only for lef1 morphants. See supplementary material Table S3 for additional information. termination were different. Therefore, it appeared unlikely that reduced proliferation was specifically responsible for the closer spacing of neuromasts and premature termination of the PLLp in lef1 morphants. Knockdown of lef1, but not rspo3, reduces the size of the lef1 expression domain As previous studies suggested that reduced proliferation following loss of lef1 function results in a reduction in the size of the leading domain of the PLLp (Valdivia et al., 2011), we compared the size of the leading domain in lef1 and rspo3 morphants, as defined by the domain of lef1 expression. As shown previously, lef1 expression was not lost in lef1 morphants (Valdivia et al., 2011; McGraw et al., 2011) (Fig. 2C,D). However, examination of the PLLp at various points along its migration (Fig. 2E) suggested that the lef1 domain is significantly smaller in lef1 morphants (Fig. 2G; supplementary material Fig. S5D). By contrast, there were no obvious changes in the size of the lef1 domain in rspo3 morphants (Fig. 2F). This suggested that a reduction in the size of the leading domain, as defined by lef1 expression, might indeed be a key change that contributes to the closer neuromast spacing and premature termination of the PLLp in lef1 morphants. It should be noted, however, that the lef1 domain did not shrink faster in lef1 morphants, it was just smaller from the beginning (Fig. 2G). lef1 knockdown is associated with slower PLLp migration prior to premature termination To determine whether differences in neuromast spacing are linked to differences in how far the PLLp migrates between deposition events, we used long-term time-lapse movies (supplementary material Movies 1-3), originally collected for cell division analysis, to compare migration speeds in lef1 and rspo3 morphants. As previously reported (Valdivia et al., 2011; McGraw et al., 2011), there was little change in the migration speed during early migration; however, the lef1 morphant PLLp became progressively slower during the later phase of migration (Fig. 3A; supplementary material Table S3). Furthermore, whereas there was no significant correlation between the migration speed and the distance migrated by the PLLp between neuromast depositions in wild-type embryos, there was a strong positive correlation in lef1 morphants (Fig. 3B,D; supplementary material Table S3). This suggests that, whereas other factors play a greater role in determining neuromast spacing in wildtype embryos, reduced speed is a significant determinant of reduced inter-neuromast spacing in lef1 morphants. However, although the PLLp migrated significantly faster in rspo3 morphants than in wild type and lef1 morphants during the L1-L2 and L2-L3 deposition cycles, there was no significant overall correlation between migration speed and the distance between neuromast depositions in rspo3 morphants (Fig. 3C; supplementary material Table S3). These
6 2392 RESEARCH ARTICLE Development 140 (11) Fig. 4. Weakening Wnt signaling facilitates proto-neuromast maturation and deposition. (A-D) Wnt inhibitors reduce lef1 expression. lef1 expression is shown in the PLLp following exposure to DMSO control (A), IWR-1 (B) and XAV939 (C), and following heat shock-induced expression of ΔNTcf (D) at 31 hpf. (E,F) IWR-1 shrinks the broad leading fgf10 expression domain while exposing more spots of trailing fgf10 expression (arrows). (G,H) Premature appearance of atoh1b expression (arrows) in IWR-1-treated PLLp at ~31 hpf. (I,J) pea3 expression is not obviously altered in IWR-1- treated embryos. (K) Proto-neuromast formation is initiated closer to the leading edge in IWR-1-treated and BCI-treated embryos. **P<0.01, ***P< Error bars indicate s.d. (L) Comparison of the relative size of the lef1 expression domain at different stages of migration in embryos treated with DMSO (red) or 10 μm IWR-1 (blue), as in Fig. 2F,G. A simple linear regression model and a paired t-test confirmed that the length of the lef1 domain is significantly smaller in the IWR-1-treated embryos. P< (M,N) Neuromast deposition in embryos treated with DMSO (M) or IWR-1 (N). results suggest that, as in wild-type embryos, factors other than migration speed play a more important role in determining spacing. Reduced Wnt activity promotes proto-neuromast maturation and deposition To determine whether changes in lef1 morphants reflect changes in the balance of Wnt and FGFR signaling, we examined how weakening the Wnt system with other manipulations would affect neuromast deposition and PLLp termination. The pharmacological Wnt signaling inhibitors IWR-1 and XAV939 stabilize Axin, which is an essential component of the β-catenin degradation complex, and reduce the amount of β-catenin available for Wnt signaling (Chen et al., 2009; Huang et al., 2009). Treatment with either IWR- 1 or XAV939 reduced the size and intensity of the leading lef1 domain in the PLLp (Fig. 4A-C; supplementary material Fig. S6A), but did not completely repress lef1 expression, as follows expression of ΔΝTcf (Aman and Piotrowski, 2008) (Fig. 4D). When examined at different points along its migration, the lef1 domain was consistently smaller in the PLLp of IWR-1-treated embryos (Fig. 4L). IWR-1 also reduced the size of the broad Wnt-dependent fgf10 domain at the leading end of the PLLp (Fig. 4E,F; supplementary material Fig. S6B), which was accompanied by the premature appearance of Atoh1a-dependent focal fgf10 expression (arrows in Fig. 4E,F; supplementary material Fig. S6D,E). Furthermore, shrinking of the Wnt active domain resulted in premature atoh1b expression, which was seen in more proto-neuromasts and closer to the leading edge of the PLLp (Fig. 4G,H; supplementary material Fig. S6G). Knockdown of lef1 had a similar effect to IWR-1 on fgf10 and atoh1b expression (supplementary material Fig. S6C,F,H). Although we did not see statistically significant differences in the size or intensity of the pea3 (etv4 Zebrafish Information Network) expression domain, as a readout of FGF signaling, in the IWR-1-treated PLLp (Fig. 4I,J; supplementary material Fig. S6I), we did observe a central accumulation of ZO- 1 (Tjp1 Zebrafish Information Network), which marks protoneuromast formation, closer to the leading end of the PLLp (Fig. 4K). In IWR-1-treated embryos, the PLLp deposited neuromasts at shorter intervals and the terminated prematurely (Fig. 4M,N). Progressively higher concentrations of IWR-1 resulted in earlier termination of the system and the deposition of fewer neuromasts (supplementary material Fig. S7). Taken together, these results suggest that reduced Wnt signaling induced by IWR-1 allows FGF signaling-dependent proto-neuromast formation closer to the leading end, reduces neuromast spacing and prematurely terminates the PLLp system, as is seen when lef1 function is compromised.
7 Lef1 regulates Dusp6 in the PLLp RESEARCH ARTICLE 2393 Fig. 5. Knockdown of fgf10 delays initial neuromast formation and deposition. (A-H) Knockdown of fgf10 delays the initial restriction of the lef1 domain, the establishment of FGFR signaling and the formation of proto-neuromasts. lef1 (A,B), pea3 (C,D), and atoh1a (E,F) expression is shown in control (conmo) and fgf10 morphant embryos at 31 hpf. For statistical analysis, see supplementary material Fig. S8. (G,H) Epithelial rosette formation visualized by central accumulation (arrows) of ZO-1 (green) and Cldnb (red). (I-P) By 35 hpf, however, there is restriction of lef1 (I,J), recovery of pea3 (K,L) and establishment of multiple proto-neuromasts, as shown by focal atoh1a (M,N) and central accumulation of ZO-1 (green) and Cldnb (red) (O,P). (Q,R) Deposition of the first neuromast is delayed in fgf10 morphants and subsequent neuromasts are deposited at closer intervals. (S) Dose-dependent delay in L1 neuromast deposition. *P<0.05, ***P< Error bars indicate s.d. (T) Comparison of the relative size of the lef1 expression domain at different stages of migration in control (red) and fgf10 morphant (blue) embryos, as in Fig. 2E. fgf10 knockdown delays the establishment of FGFR signaling and the shrinking of the lef1 domain We next asked whether weakening the FGF system, by knocking down fgf3 or fgf10, delays neuromast formation and deposition. Whereas knockdown of fgf3 had little effect on pea3 expression (Lecaudey et al., 2008; Nechiporuk and Raible, 2008) (data not shown), knockdown of fgf10 was associated with an expanded lef1 domain and a failure to establish robust pea3 expression at 31 hpf (Fig. 5A-D; supplementary material Fig. S8A,B). By 35 hpf, however, pea3 expression was well established in the trailing domain and lef1 had become restricted to a much smaller leading domain, as in control embryos (Fig. 5I-L). fgf10 knockdown delays initial proto-neuromast formation and deposition The delay in establishing FGFR signaling in fgf10 morphants was accompanied by the absence of atoh1a expression in the leading domain of the PLLp at 31 hpf (Fig. 5E,F) (Nechiporuk and Raible, 2008). This is likely to reflect a delay in the initial formation of protoneuromasts, which, in the wild-type PLLp, starts at the trailing end and then proceeds sequentially toward the leading end (Nechiporuk and Raible, 2008). Consistent with this interpretation, absence of atoh1a expression was accompanied by a severe delay in the accumulation of ZO-1 and Cldnb in the fgf10 morphant PLLp (Fig. 5G,H). Once initiated, periodic proto-neuromast formation in the fgf10 morphant PLLp progressed from the trailing end toward the leading end of the PLLp, as in wild type. This was marked by the appearance of restricted atoh1a expression and the accumulation of ZO-1 and Cldnb in more leading domains of the PLLp (Fig. 5M-P). The neuromast formation and deposition rate correlates with shrinking of the lef1 domain Delay in proto-neuromast formation in fgf10 morphants was accompanied by a dose-dependent delay in deposition of the first
8 2394 RESEARCH ARTICLE Development 140 (11) Fig. 6. Attenuation of Wnt signaling facilitates neuromast formation in fgf10 morphants. (A-L) Attenuation of Wnt signaling with IWR-1 facilitates earlier shrinking of the Wnt system, earlier establishment of effective FGFR signaling and earlier initiation of neuromast deposition in the PLLp of fgf10 morphants. lef1 expression (A-D), pea3 expression (E-H) and the neuromast deposition pattern (I-L, arrows) are shown in control morphants (A,E,I), control morphants with IWR-1 treatment (B,F,J), fgf10 morphants (C,G,K) and fgf10 morphants with IWR-1 treatment (D,H,L). For statistical analysis, see supplementary material Figs S12, S13. stable neuromast (Fig. 5Q-S; supplementary material Fig. S9). However, after L1 neuromast deposition, subsequent neuromasts were deposited closer to each other (Fig. 5Q,R; supplementary material Fig. S9). The initial delay and subsequent recovery of neuromast deposition correlated with an initial delay in the restriction of the lef1 domain and a subsequent recovery in shrinking of the lef1 domain in the fgf10 morphant PLLp (Fig. 5A,B,I,J,T). Although these changes were not seen in fgf10 mutants (Lecaudey et al., 2008), the effects of fgf10 knockdown on PLLp development were confirmed with two independent previously characterized MOs [fgf10-mo1 (Nechiporuk and Raible, 2008) and fgf10a-mo2 (Norton et al., 2005)] (supplementary material Fig. S10). Furthermore, by comparing the effects of knockdown of fgf10a, fgf10b, or both in combination, we confirmed that these effects are specific to fgf10a (data not shown). Time-lapse imaging showed that PLLp migration was significantly slower in fgf10 morphants between the deposition of the L2 and L3 neuromasts; however, as in wild-type embryos, there was no overall significant correlation between migration speed and distance traveled between neuromast depositions (supplementary material Movie 4, Fig. S11). This suggests that reduced speed is not the primary factor that determines the closer spacing of deposited neuromasts in fgf10 morphants, and that other factors appear to play a more important role. The balance of Wnt and FGF signaling determines the initiation of proto-neuromast formation Progressive shrinking of the lef1 expression domain in the PLLp could be a secondary effect of sequential proto-neuromast formation from progenitors in the leading domain that lose lef1 expression as they are incorporated into proto-neuromasts; alternatively, it could reflect attenuation of the Wnt system, which serves as a precondition for effective proto-neuromast formation. Delay in formation of the first stable proto-neuromast in fgf10 morphants is associated with persistent broad lef1 expression. If the expanded lef1 expression simply reflects failure of proto-neuromast formation due to ineffective FGF signaling, then attenuation of Wnt signaling would not be expected to have any effect on when proto-neuromast formation is initiated in this context. If, however, shrinking of the Wnt active domain defines a pre-condition for effective FGFdependent proto-neuromast formation, then attenuating Wnt signaling should facilitate earlier proto-neuromast formation in the fgf10 morphant PLLp. Weakening the Wnt system with IWR-1 in fgf10 morphants facilitated earlier shrinking of the lef1 expression domain (Fig. 6A- D; supplementary material Fig. S12A,B). This was associated with earlier establishment of the trailing FGF system, as indicated by pea3 expression (Fig. 6E-H; supplementary material Fig. S12C), and earlier deposition of the first neuromast (Fig. 6I-L; supplementary material Fig. S13A,B). Similarly, knockdown of lef1 in fgf10 morphants facilitated earlier deposition of the first neuromast (supplementary material Fig. S13C,D). Together, these observations support our interpretation that the dynamic balance of Wnt and FGFR signaling, in some way regulated by lef1, influences the initiation of proto-neuromast formation in the PLLp. Lef1 is responsible for expression of dusp6 in the leading Wnt active zone Next, we asked whether Lef1 influences the balance of Wnt and FGF signaling by regulating the expression of factors that either facilitate Wnt or inhibit FGF signaling. Previous studies have shown that Lef1 is not essential for driving its own expression nor is it required for expression of Sef, an inhibitor of FGFR signaling in the leading domain (McGraw et al., 2011; Valdivia et al., 2011). However, we found that Lef1 is required for the expression of dusp6 [previously known as map kinase phosphatase 3 (mkp3)], which encodes a member of the dual specificity protein phosphatase subfamily that functions as an intracellular inhibitor of ERK signaling (Tsang and Dawid, 2004). Unlike sef, the Wnt-dependent expression of which is more restricted in the leading compartment
9 Lef1 regulates Dusp6 in the PLLp RESEARCH ARTICLE 2395 Fig. 7. Lef1 determines leading dusp6 expression and inhibition of Lef1 function influences the pattern of proto-neuromast formation and neuromast deposition. (A-D) dusp6 expression is regulated by Lef1 and FGFR signaling. (A) dusp6 is expressed in the leading two-thirds of the PLLp. (B) lef1 knockdown eliminates dusp6 expression in a leading domain. (C) SU5402 treatment eliminates trailing dusp6 expression. (D) SU5402 treatment in lef1 morphants eliminates dusp6 expression in the PLLp. Bracket shows where dusp6 expression is lost. Asterisk indicates persistent dusp6 expression in prospective inter-neuromast cells. (E,F) pea3 expression shows that FGFR signaling is enhanced in BCI-treated embryos. For statistical analysis, see supplementary material Fig. S15B. (G,H) lef1 expression suggests that Wnt signaling is not obviously altered in BCI-treated embryos. For statistical analysis, see supplementary material Fig. S15A. (I,J) atoh1b expression is seen in more proto-neuromasts closer to the lead edge of the PLLp. For statistical analysis, see supplementary material Fig. S6E. (K,L) Neuromasts are deposited closer together in BCI-treated embryos. For statistical analysis, see supplementary material Fig. S16. (M) There is no apparent difference between DMSO-treated controls and BCI-treated embryos in the size of the lef1 domain over the course of PLLp migration. (N) mcherry or Dusp6-mCherry expression was induced by heat shock treatment during PLLp migration. After the PLLp had completed migration, the numbers of red fluorescent cells were counted in each neuromast (see supplementary material Fig. S17). Cells expressing Dusp6-mCherry were less likely to be deposited in neuromasts L1-4 and more likely to be deposited in L5-6 or terminal neuromasts of the PLLp. **P<0.01. Error bars indicate s.d. of the PLLp (Aman and Piotrowski, 2008), dusp6 is expressed in approximately the leading two-thirds of the PLLp (Fig. 7A). In lef1 morphants there is a specific loss of dusp6 expression in the leading end, while some expression in the trailing end is retained (Fig. 7B). When embryos were exposed to the FGF signaling inhibitor SU5402, trailing expression was lost, whereas expression in the leading zone was retained (Fig. 7C). When embryos were both injected with lef1 MO and exposed to SU5402, dusp6 expression was lost throughout the PLLp (Fig. 7D). These observations suggest that dusp6 expression is under the dual control of lef1-dependent Wnt signaling in the leading zone and FGF signaling in the trailing zone. Dual regulation of dusp6 by Wnt and FGF signaling has been described previously in zebrafish late gastrula embryos (Tsang and Dawid, 2004). It should be noted, however, that exposure to SU5402 resulted in a specific loss of dusp6 in the trailing domain, in spite of the fact that inhibition of FGF signaling causes an expansion of Wnt-dependent lef1 expression to the trailing end (Aman and Piotrowski, 2008). Restricted expression of dusp6 in the leading zone following exposure to SU5402 suggests that some additional factors regulate dusp6 expression in the PLLp. Knockdown of rspo3 did not alter the expression of dusp6 (supplementary material Fig. S14). Inhibition of Dusp6 function facilitates neuromast formation and deposition If loss of lef1-dependent dusp6 expression is responsible for closer neuromast deposition, inhibition of dusp6 itself should cause similar changes. To test this, we used a specific chemical inhibitor of Dusp6, BCI (Molina et al., 2009). Consistent with the function of Dusp6 as an inhibitor of FGF signaling, BCI treatment increased FGF signaling-dependent pea3 expression in the PLLp (Fig. 7E,F; supplementary material Fig. S15B). It also facilitated protoneuromast formation and maturation: central ZO-1 accumulation was first seen closer to the leading edge of the PLLp (Fig. 4K) and atoh1b expression was prematurely induced in the trailing part of the PLLp (Fig. 7I,J; supplementary material Fig. S6G). In addition,
10 2396 RESEARCH ARTICLE Development 140 (11) exposure to BCI reduced neuromast spacing and led to premature termination of PLLp migration (Fig. 7K,L; supplementary material Fig. S16), as in lef1 morphants. However, unlike lef1 morphants, the lef1 domain was not consistently smaller in BCI-treated embryos (Fig. 7G,H,M). This is consistent with Dusp6 functioning downstream of lef1 and with changes in Dusp6 function not feeding back on lef1 expression. As inhibition of Dusp6 with BCI facilitated proto-neuromast formation, we asked whether induced expression of Dusp6 would reduce the probability of cells being incorporated into protoneuromasts and being deposited by the migrating PLLp. Plasmids encoding heat shock-inducible mcherry red fluorescent protein or Dusp6 in conjunction with mcherry were injected into 1- to 2-cell stage tg[cldnb:lyngfp] embryos. Embryos were heat-treated at 38 C for 45 minutes at 24, 30 and 36 hpf to maintain induced expression of mcherry or Dusp6-mCherry. The distribution of mcherry + or Dusp6-mCherry + cells in deposited neuromasts or terminal neuromasts of the PLLp was compared at 56 hpf (supplementary material Fig. S17). For effective statistical comparison of their relative distribution, only embryos with at least ten positive cells in the PLLp system were counted. Cells with induced expression of Dusp6 were significantly less likely to be deposited in neuromasts L1-4 and more likely to be deposited in L5-6 or terminal neuromasts of the PLLp (Fig. 7N). Together, these observations show that Lef1 determines the expression of Dusp6 in a leading domain of the PLLp and that Lef1-dependent Dusp6 expression regulates FGF signaling-dependent incorporation of progenitors into proto-neuromasts. DISCUSSION This study began with an examination of Rspo3 function in the PLLp. It has shown that, like Lef1, Rspo3 determines Wntdependent proliferation in the PLLp, and that Rspo3 loss results in a pattern of reduced proliferation similar to that seen following loss of Lef1 function. It was initially thought that reduced proliferation could account for both the premature termination of the PLLp system and the closer spacing of deposited neuromasts when Lef1 function is lost. However, our studies suggest that these changes are more likely to be related to an additional role of lef1 in determining dusp6 expression and in influencing where FGF signaling can effectively determine the morphogenesis of nascent protoneuromasts. Our observations reveal how Wnt signaling, acting via distinct target genes, simultaneously promotes proliferation and inhibits proto-neuromast formation, ensuring that both processes are coordinated in the migrating PLLp. Our study shows that the balance of Wnt and FGF signaling influences when and where proto-neuromasts form and that this can influence the pattern of neuromast deposition by the migrating PLLp. Previous studies have suggested that Sef, which broadly inhibits FGF signaling by interfering with the function of the FGF receptor, is expressed in response to Wnt signaling in a leading domain (Aman and Piotrowski, 2008). We have now shown that Dusp6, a phosphatase that inhibits ERK signaling, is expressed in response to Lef1-mediated Wnt/β-catenin signaling in the leading domain of the PLLp. This is the branch of the FGF signaling pathway that is specifically responsible for mediating morphogenetic changes involved in neuromast formation (Harding and Nechiporuk, 2012). The sequential formation of proto-neuromasts is accompanied by the complementary progressive restriction of lef1 expression to a smaller leading domain of the PLLp. This can be interpreted in a number of ways. The leading lef1 domain can be thought to represent a progenitor population from which cells are progressively lost as they are incorporated into neuromasts. From this perspective, the shrinking of the lef1 domain is a secondary consequence of proto-neuromast formation/differentiation. Alternatively, reduction of Wnt signaling in response to FGF signaling might serve as a precondition for effective proto-neuromast formation in the trailing domain. In this context, shrinking of the lef1 expression domain may indirectly reflect the dynamic tug-of-war between the Wnt and FGF signaling pathways that determines when and how close to the leading edge the formation of proto-neuromasts is allowed. We have shown that knockdown of fgf10 delays the establishment of a robust FGF signaling system and consequently delays the formation and deposition of stable neuromasts. In this context, weakening the Wnt system with IWR-1 or by knockdown of the Wnt effector lef1 allows the earlier formation of proto-neuromasts, supporting our interpretation that the balance of Wnt and FGF signaling determines when proto-neuromasts are formed. Weakening the Wnt system or loss of lef1 results in both a shorter leading lef1 expression domain and the formation of nascent protoneuromasts closer to the leading end. But how is the shorter leading lef1 expression domain linked to the shorter distance between deposited neuromasts? Inhibition of Dusp6 function results in many changes similar to those seen when lef1 function is lost: the spacing between deposited neuromasts is closer and nascent protoneuromasts are formed closer to the leading edge of the migrating PLLp. Nevertheless, there is no obvious reduction in the size of the leading domain as revealed by lef1 expression. This suggests that reduction in the length of the leading lef1 domain might not be directly related to changes in spacing between deposited neuromasts. What might be more relevant is the reduction in the size of the domain between the most recently formed protoneuromast and the leading edge of the PLLp, as this is clearly smaller in lef1-deficient, IWR-1-treated and Dusp6-inhibited embryos. We have also shown that the spacing of deposited neuromasts correlates with the migration speed between deposition events in lef1 morphants. This suggests that the size of the leading protoneuromast-free domain might influence the PLLp migration speed, but how remains unclear. One possibility is that it may be related to changes in cell behavior and morphology that accompany nascent proto-neuromast formation; the relatively mesenchymal leading cells might become progressively less migratory as they form epithelial rosettes. In this manner, the size of the proto-neuromastfree leading domain may determine the efficacy of PLLp migration. Preliminary studies suggest that there is also an expansion of cxcr7b expression and a reduction of the leading cxcr7b-free domain in the PLLp of lef1 morphants and BCI-treated or IWR-1 treated embryos (supplementary material Fig. S18). As initiation of cxcr7b expression has been suggested to correlate with cells preparing for deposition (Aman et al., 2011), this too might contribute to slower PLLp migration. However, the mechanism by which potential changes in cxcr7b expression and the formation of proto-neuromasts closer to the leading end influence migration speed remains unclear and will be addressed in future studies. The initially broad lef1 domain progressively shrinks as the PLLp migrates from the ear to the tip of the tail, where its eventual demise terminates the PLLp system. Inhibiting lef1 function facilitates the premature loss of the lef1 domain and termination of the PLLp system when it has only completed about half its journey to the tip of the tail. Although there is significant variation in the precise pattern of neuromast deposition, the shrinking and eventual demise of the lef1 domain are tightly linked to its arrival near the tip of the
Figure S1a. The modular dcas9 fusion system works efficiently to suppress and activate endogenous gene expression in C. elegans
 Figure S1a. The modular dcas9 fusion system works efficiently to suppress and activate endogenous gene expression in C. elegans A, a normal wild-type hermaphrodite N2 is shown. B, a dpy-5-supressed F1
Figure S1a. The modular dcas9 fusion system works efficiently to suppress and activate endogenous gene expression in C. elegans A, a normal wild-type hermaphrodite N2 is shown. B, a dpy-5-supressed F1
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl
 Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Supplementary Fig. S1. Schematic representation of mouse lines Pax6 fl/fl and mrx-cre used in this study. (A) To generate Pax6 fl/ fl, loxp sites flanking exons 3-6 (red arrowheads) were introduced into
Supplemental Information
 Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Fig. S1. Molecular phylogenetic analysis of AtHD-ZIP IV family. A phylogenetic tree was constructed using Bayesian analysis with Markov Chain Monte
 Fig. S1. Molecular phylogenetic analysis of AtHD-ZIP IV family. A phylogenetic tree was constructed using Bayesian analysis with Markov Chain Monte Carlo algorithm for one million generations to obtain
Fig. S1. Molecular phylogenetic analysis of AtHD-ZIP IV family. A phylogenetic tree was constructed using Bayesian analysis with Markov Chain Monte Carlo algorithm for one million generations to obtain
Supplementary Figure 1. Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings.
 Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Nature Biotechnology: doi: /nbt.4166
 Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and
 Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
Supporting Information
 Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Supplementary Information. Isl2b regulates anterior second heart field development in zebrafish
 Supplementary Information Isl2b regulates anterior second heart field development in zebrafish Hagen R. Witzel 1, Sirisha Cheedipudi 1, Rui Gao 1, Didier Y.R. Stainier 2 and Gergana D. Dobreva 1,3* 1 Origin
Supplementary Information Isl2b regulates anterior second heart field development in zebrafish Hagen R. Witzel 1, Sirisha Cheedipudi 1, Rui Gao 1, Didier Y.R. Stainier 2 and Gergana D. Dobreva 1,3* 1 Origin
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
7.22 Final Exam points
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Supplementary Figure 1. Wnt10a mutation causes aberrant molar tooth development and formation of an ectopic M4 molar. (a,b) Smaller molar teeth with
 Supplementary Figure 1. Wnt10a mutation causes aberrant molar tooth development and formation of an ectopic M4 molar. (a,b) Smaller molar teeth with blunted cusp formation, and presence of an ectopic molar
Supplementary Figure 1. Wnt10a mutation causes aberrant molar tooth development and formation of an ectopic M4 molar. (a,b) Smaller molar teeth with blunted cusp formation, and presence of an ectopic molar
SUPPLEMENTARY INFORMATION
 a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
embryos. Asterisk represents loss of or reduced expression. Brackets represent
 Supplemental Figures Supplemental Figure 1. tfec expression is highly enriched in tail endothelial cells (A- B) ISH of tfec at 15 and 16hpf in WT embryos. (C- D) ISH of tfec at 36 and 38hpf in WT embryos.
Supplemental Figures Supplemental Figure 1. tfec expression is highly enriched in tail endothelial cells (A- B) ISH of tfec at 15 and 16hpf in WT embryos. (C- D) ISH of tfec at 36 and 38hpf in WT embryos.
Fig Hypoxia causes growth retardation and developmental delay. (A) Morphology of wildtype zebrafish embryos at 48 hours post fertilization
 FIGURES AND TABLES Fig. 1-1. Hypoxia causes growth retardation and developmental delay. (A) Morphology of wildtype zebrafish embryos at 48 hours post fertilization (hpf) after 24 h of normoxia or hypoxia
FIGURES AND TABLES Fig. 1-1. Hypoxia causes growth retardation and developmental delay. (A) Morphology of wildtype zebrafish embryos at 48 hours post fertilization (hpf) after 24 h of normoxia or hypoxia
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product.
 Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
To assess the localization of Citrine fusion proteins, we performed antibody staining to
 Trinh et al 1 SUPPLEMENTAL MATERIAL FlipTraps recapitulate endogenous protein localization To assess the localization of Citrine fusion proteins, we performed antibody staining to compare the expression
Trinh et al 1 SUPPLEMENTAL MATERIAL FlipTraps recapitulate endogenous protein localization To assess the localization of Citrine fusion proteins, we performed antibody staining to compare the expression
A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish
 Developmental Cell Supplemental Information A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish Julien Ablain, Ellen M. Durand, Song Yang, Yi Zhou, and Leonard I. Zon % larvae
Developmental Cell Supplemental Information A CRISPR/Cas9 Vector System for Tissue-Specific Gene Disruption in Zebrafish Julien Ablain, Ellen M. Durand, Song Yang, Yi Zhou, and Leonard I. Zon % larvae
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3363 Supplementary Figure 1 Several WNTs bind to the extracellular domains of PKD1. (a) HEK293T cells were co-transfected with indicated plasmids. Flag-tagged proteins were immunoprecipiated
DOI: 10.1038/ncb3363 Supplementary Figure 1 Several WNTs bind to the extracellular domains of PKD1. (a) HEK293T cells were co-transfected with indicated plasmids. Flag-tagged proteins were immunoprecipiated
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
Supplemental Figure 1 (Figure S1), related to Figure 1 Figure S1 provides evidence to demonstrate Nfatc1Cre is a mouse line that directed gene
 Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
Developmental Cell, Volume 25 Supplemental Information Brg1 Governs a Positive Feedback Circuit in the Hair Follicle for Tissue Regeneration and Repair Yiqin Xiong, Wei Li, Ching Shang, Richard M. Chen,
Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin
 Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Supplementary Information 1 Quantitative real-time RT-PCR analysis of the expression levels of E-cadherin and ribosomal protein L19 (RPL19) mrna in cleft and bud epithelial cells of embryonic salivary
Revised: RG-RV2 by Fukuhara et al.
 Supplemental Figure 1 The generation of Spns2 conditional knockout mice. (A) Schematic representation of the wild type Spns2 locus (Spns2 + ), the targeted allele, the floxed allele (Spns2 f ) and the
Supplemental Figure 1 The generation of Spns2 conditional knockout mice. (A) Schematic representation of the wild type Spns2 locus (Spns2 + ), the targeted allele, the floxed allele (Spns2 f ) and the
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Apoptosis analyses and quantification. Activated caspase3 immunostaining on sections of E9.0 Tcof1 +/ -;p53 +/- (a) and Tcof1 +/ -;p53 -/- (b) littermate embryos
Supplementary Figures Supplementary Figure 1. Apoptosis analyses and quantification. Activated caspase3 immunostaining on sections of E9.0 Tcof1 +/ -;p53 +/- (a) and Tcof1 +/ -;p53 -/- (b) littermate embryos
sides of the aleurone (Al) but it is excluded from the basal endosperm transfer layer
 Supplemental Data. Gómez et al. (2009). The maize transcription factor MRP-1 (Myb-Related-Protein-1) is a key regulator of the differentiation of transfer cells. Supplemental Figure 1. Expression analyses
Supplemental Data. Gómez et al. (2009). The maize transcription factor MRP-1 (Myb-Related-Protein-1) is a key regulator of the differentiation of transfer cells. Supplemental Figure 1. Expression analyses
Supplementary Information. c d e
 Supplementary Information a b c d e f Supplementary Figure 1. atabcg30, atabcg31, and atabcg40 mutant seeds germinate faster than the wild type on ½ MS medium supplemented with ABA (a and d-f) Germination
Supplementary Information a b c d e f Supplementary Figure 1. atabcg30, atabcg31, and atabcg40 mutant seeds germinate faster than the wild type on ½ MS medium supplemented with ABA (a and d-f) Germination
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured
 Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
Supplementary Figure 1. Co-localization of GLUT1 and DNAL4 in BeWo cells cultured under static conditions. Cells were seeded in the chamber area of the device and cultured overnight without medium perfusion.
Supplementary Figure 1. Phenotype, morphology and distribution of embryonic GFP +
 Supplementary Figure 1 Supplementary Figure 1. Phenotype, morphology and distribution of embryonic GFP + haematopoietic cells in the intestine. GFP + cells from E15.5 intestines were obtained after collagenase
Supplementary Figure 1 Supplementary Figure 1. Phenotype, morphology and distribution of embryonic GFP + haematopoietic cells in the intestine. GFP + cells from E15.5 intestines were obtained after collagenase
Supplemental Information. Boundary Formation through a Direct. Threshold-Based Readout. of Mobile Small RNA Gradients
 Developmental Cell, Volume 43 Supplemental Information Boundary Formation through a Direct Threshold-Based Readout of Mobile Small RNA Gradients Damianos S. Skopelitis, Anna H. Benkovics, Aman Y. Husbands,
Developmental Cell, Volume 43 Supplemental Information Boundary Formation through a Direct Threshold-Based Readout of Mobile Small RNA Gradients Damianos S. Skopelitis, Anna H. Benkovics, Aman Y. Husbands,
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
(a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for
 Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Aquatic Vertebrates Platform Services (See services description below, page 3)
 Aquatic Vertebrates Platform Services (See services description below, page 3) Services 1. Micro-injection of DNA, morpholino or mrna in zebrafish and medaka embryos. 2. Generation of stable zebrafish
Aquatic Vertebrates Platform Services (See services description below, page 3) Services 1. Micro-injection of DNA, morpholino or mrna in zebrafish and medaka embryos. 2. Generation of stable zebrafish
Supporting Information
 Supporting Information Ho et al. 10.1073/pnas.0808899106 SI Materials and Methods In immunostaining, antibodies from Developmental Studies Hybridoma ank were -FasII (mouse, 1:200), - (mouse, 1:100), and
Supporting Information Ho et al. 10.1073/pnas.0808899106 SI Materials and Methods In immunostaining, antibodies from Developmental Studies Hybridoma ank were -FasII (mouse, 1:200), - (mouse, 1:100), and
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15
 Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
Supplementary Table, Figures and Videos
 Supplementary Table, Figures and Videos Table S1. Oligonucleotides used for different approaches. (A) RT-qPCR study. (B) qpcr study after ChIP assay. (C) Probes used for EMSA. Figure S1. Notch activation
Supplementary Table, Figures and Videos Table S1. Oligonucleotides used for different approaches. (A) RT-qPCR study. (B) qpcr study after ChIP assay. (C) Probes used for EMSA. Figure S1. Notch activation
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Paul et al.,
 Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency.
 Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency. (a) Transfection of different concentration of GAS5-overexpressing
Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency. (a) Transfection of different concentration of GAS5-overexpressing
Name AP Biology Mrs. Laux Take home test #11 on Chapters 14, 15, and 17 DUE: MONDAY, DECEMBER 21, 2009
 MULTIPLE CHOICE QUESTIONS 1. Inducible genes are usually actively transcribed when: A. the molecule degraded by the enzyme(s) is present in the cell. B. repressor molecules bind to the promoter. C. lactose
MULTIPLE CHOICE QUESTIONS 1. Inducible genes are usually actively transcribed when: A. the molecule degraded by the enzyme(s) is present in the cell. B. repressor molecules bind to the promoter. C. lactose
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12119 SUPPLEMENTARY FIGURES AND LEGENDS pre-let-7a- 1 +14U pre-let-7a- 1 Ddx3x Dhx30 Dis3l2 Elavl1 Ggt5 Hnrnph 2 Osbpl5 Puf60 Rnpc3 Rpl7 Sf3b3 Sf3b4 Tia1 Triobp U2af1 U2af2 1 6 2 4 3
doi:10.1038/nature12119 SUPPLEMENTARY FIGURES AND LEGENDS pre-let-7a- 1 +14U pre-let-7a- 1 Ddx3x Dhx30 Dis3l2 Elavl1 Ggt5 Hnrnph 2 Osbpl5 Puf60 Rnpc3 Rpl7 Sf3b3 Sf3b4 Tia1 Triobp U2af1 U2af2 1 6 2 4 3
Supplementary Figure 1. jmj30-2 and jmj32-1 produce null mutants. (a) Schematic drawing of JMJ30 and JMJ32 genome structure showing regions amplified
 Supplementary Figure 1. jmj30-2 and jmj32-1 produce null mutants. (a) Schematic drawing of JMJ30 and JMJ32 genome structure showing regions amplified by primers used for mrna expression analysis. Gray
Supplementary Figure 1. jmj30-2 and jmj32-1 produce null mutants. (a) Schematic drawing of JMJ30 and JMJ32 genome structure showing regions amplified by primers used for mrna expression analysis. Gray
Supplementary Figure 1. Confirmation of sirna in PC3 and H1299 cells PC3 (a) and H1299 (b) cells were transfected with sirna oligonucleotides
 Supplementary Figure 1. Confirmation of sirna in PC3 and H1299 cells PC3 (a) and H1299 (b) cells were transfected with sirna oligonucleotides targeting RCP (SMARTPool (RCP) or two individual oligos (RCP#1
Supplementary Figure 1. Confirmation of sirna in PC3 and H1299 cells PC3 (a) and H1299 (b) cells were transfected with sirna oligonucleotides targeting RCP (SMARTPool (RCP) or two individual oligos (RCP#1
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Methods
 Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
DOI:.38/ncb327 a b Sequence coverage (%) 4 3 2 IP: -GFP isoform IP: GFP IP: -GFP IP: GFP Sequence coverage (%) 4 3 2 IP: -GFP IP: GFP 33 52 58 isoform 2 33 49 47 IP: Control IP: Peptide Sequence Start
SureSilencing sirna Array Technology Overview
 SureSilencing sirna Array Technology Overview Pathway-Focused sirna-based RNA Interference Topics to be Covered Who is SuperArray? Brief Introduction to RNA Interference Challenges Facing RNA Interference
SureSilencing sirna Array Technology Overview Pathway-Focused sirna-based RNA Interference Topics to be Covered Who is SuperArray? Brief Introduction to RNA Interference Challenges Facing RNA Interference
Figure S1 Glucocorticoid receptor-green fluorescent protein (GR-GFP) reporter construct.
 Figure S1 Glucocorticoid receptor-green fluorescent protein (GR-GFP) reporter construct. (A) Schematic display of the construct is presented detailing binding sites and regions of interest within the GR
Figure S1 Glucocorticoid receptor-green fluorescent protein (GR-GFP) reporter construct. (A) Schematic display of the construct is presented detailing binding sites and regions of interest within the GR
Supplemental Figure 1 HDA18 has an HDAC domain and therefore has concentration dependent and TSA inhibited histone deacetylase activity.
 Supplemental Figure 1 HDA18 has an HDAC domain and therefore has concentration dependent and TSA inhibited histone deacetylase activity. (A) Amino acid alignment of HDA5, HDA15 and HDA18. The blue line
Supplemental Figure 1 HDA18 has an HDAC domain and therefore has concentration dependent and TSA inhibited histone deacetylase activity. (A) Amino acid alignment of HDA5, HDA15 and HDA18. The blue line
Figure S1, related to Figure 1. Characterization of biosensor behavior in vivo.
 Developmental Cell, Volume 23 Supplemental Information Separase Biosensor Reveals that Cohesin Cleavage Timing Depends on Phosphatase PP2A Cdc55 Regulation Gilad Yaakov, Kurt Thorn, and David O. Morgan
Developmental Cell, Volume 23 Supplemental Information Separase Biosensor Reveals that Cohesin Cleavage Timing Depends on Phosphatase PP2A Cdc55 Regulation Gilad Yaakov, Kurt Thorn, and David O. Morgan
Supplementary Information. Conversion of vascular endothelial cells into multipotent stem-like cells
 Supplementary Information Conversion of vascular endothelial cells into multipotent stem-like cells Damian Medici 1, Eileen M. Shore 2,3,4, Vitali Y. Lounev 2,4, Frederick S. Kaplan 2,4,5, Raghu Kalluri
Supplementary Information Conversion of vascular endothelial cells into multipotent stem-like cells Damian Medici 1, Eileen M. Shore 2,3,4, Vitali Y. Lounev 2,4, Frederick S. Kaplan 2,4,5, Raghu Kalluri
Supplementary Figure 1 A
 Supplementary Figure A B M. mullata p53, 3 UTR Luciferase activity (%) mir-5b 8 Le et al. Supplementary Information NC-DP - + - - - - - NC-DP - - + - - - - NC-DP3 - - - + - - - 5b-DP - - - - + + + NC-AS
Supplementary Figure A B M. mullata p53, 3 UTR Luciferase activity (%) mir-5b 8 Le et al. Supplementary Information NC-DP - + - - - - - NC-DP - - + - - - - NC-DP3 - - - + - - - 5b-DP - - - - + + + NC-AS
b number of motif occurrences
 supplementary information DOI: 1.138/ncb194 a b number of motif occurrences number of motif occurrences number of motif occurrences I II III IV V X TTGGTCAGTGCA 3 8 23 4 13 239 TTGGTCAGTGCT 7 5 8 1 3 83
supplementary information DOI: 1.138/ncb194 a b number of motif occurrences number of motif occurrences number of motif occurrences I II III IV V X TTGGTCAGTGCA 3 8 23 4 13 239 TTGGTCAGTGCT 7 5 8 1 3 83
Practice Problems 5. Location of LSA-GFP fluorescence
 Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Bi 1x Spring 2016: LacI Titration
 Bi 1x Spring 2016: LacI Titration 1 Overview In this experiment, you will measure the effect of various mutated LacI repressor ribosome binding sites in an E. coli cell by measuring the expression of a
Bi 1x Spring 2016: LacI Titration 1 Overview In this experiment, you will measure the effect of various mutated LacI repressor ribosome binding sites in an E. coli cell by measuring the expression of a
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table.
 Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
7.22 Example Problems for Exam 1 The exam will be of this format. It will consist of 2-3 sets scenarios.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Supplementary Information Alternative splicing of CD44 mrna by ESRP1 enhances lung colonization of metastatic cancer cell
 Supplementary Information Alternative splicing of CD44 mrna by ESRP1 enhances lung colonization of metastatic cancer cell Supplementary Figures S1-S3 Supplementary Methods Supplementary Figure S1. Identification
Supplementary Information Alternative splicing of CD44 mrna by ESRP1 enhances lung colonization of metastatic cancer cell Supplementary Figures S1-S3 Supplementary Methods Supplementary Figure S1. Identification
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling
 Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1245533/dc1 Supplementary Materials for A Mechanism for Reorientation of Cortical Microtubule Arrays Driven by Microtubule Severing Jelmer J. Lindeboom, Masayoshi
www.sciencemag.org/cgi/content/full/science.1245533/dc1 Supplementary Materials for A Mechanism for Reorientation of Cortical Microtubule Arrays Driven by Microtubule Severing Jelmer J. Lindeboom, Masayoshi
Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and
 Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
Determining positive selection gates for LRCs and nonlrcs Positive selection gates for the collection of LRCs or nonlrcs had to be drawn based on the location and shape of the Gaussian distributions. For
over time using live cell microscopy. The time post infection is indicated in the lower left corner.
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Supplementary Movie 1 Description: Fusion of NBs. BSR cells were infected
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Supplementary Movie 1 Description: Fusion of NBs. BSR cells were infected
Two classes of silencing RNAs move between Caenorhabditis elegans tissues.
 Two classes of silencing RNAs move between Caenorhabditis elegans tissues. Antony M Jose, Giancarlo A Garcia, and Craig P Hunter. Supplementary Figures, Figure Legends, and Tables. Supplementary Figure
Two classes of silencing RNAs move between Caenorhabditis elegans tissues. Antony M Jose, Giancarlo A Garcia, and Craig P Hunter. Supplementary Figures, Figure Legends, and Tables. Supplementary Figure
Marilyn G. Rimando, Hao-Hsiang Wu, Yu-An Liu, Chien-Wei Lee, Shu-Wen Kuo, Yin-
 Supplementary Information Glucocorticoid receptor and Histone Deacetylase 6 mediate the differential effect of dexamethasone during osteogenesis of Mesenchymal stromal cells (MSCs) Marilyn G. Rimando,
Supplementary Information Glucocorticoid receptor and Histone Deacetylase 6 mediate the differential effect of dexamethasone during osteogenesis of Mesenchymal stromal cells (MSCs) Marilyn G. Rimando,
SUPPLEMENTARY INFORMATION
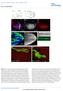 DOI: 10.1038/ncb2806 Supplemental Material: Figure S1 Wnt4 PCP phenotypes are not mediated through canonical Wnt signaling. (a-a ) Illustration of wg, dwnt4 and dwnt6 expression patterns in wing (a) and
DOI: 10.1038/ncb2806 Supplemental Material: Figure S1 Wnt4 PCP phenotypes are not mediated through canonical Wnt signaling. (a-a ) Illustration of wg, dwnt4 and dwnt6 expression patterns in wing (a) and
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
Flow cytometry Stained cells were analyzed and sorted by SORP FACS Aria (BD Biosciences).
 Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
Mice C57BL/6-Ly5.1 or -Ly5.2 congenic mice were used for LSK transduction and competitive repopulation assays. Animal care was in accordance with the guidelines of Keio University for animal and recombinant
bronchial epithelial cells (I). Bronchi are outlined with dashed line. Scale bars = 25 µm, if not
 Supplemental Figure S1: ronchial epithelial cell polarity and integrity is maintained in bronchi. (A-E) Staining for selected markers of bronchial cell differentiation and intracellular compartments is
Supplemental Figure S1: ronchial epithelial cell polarity and integrity is maintained in bronchi. (A-E) Staining for selected markers of bronchial cell differentiation and intracellular compartments is
Nature Immunology: doi: /ni.3694
 Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Supplementary Figures and Legends.
 Supplementary Figures and Legends. Supplementary Figure 1: Impact of injury on Rb1 and PPARϒ expression. Following ipsilateral axotomy injury, adult DRG expression of Rb1 mrna declined (*p
Supplementary Figures and Legends. Supplementary Figure 1: Impact of injury on Rb1 and PPARϒ expression. Following ipsilateral axotomy injury, adult DRG expression of Rb1 mrna declined (*p
Figure S2. Response of mouse ES cells to GSK3 inhibition. Mentioned in discussion
 Stem Cell Reports, Volume 1 Supplemental Information Robust Self-Renewal of Rat Embryonic Stem Cells Requires Fine-Tuning of Glycogen Synthase Kinase-3 Inhibition Yaoyao Chen, Kathryn Blair, and Austin
Stem Cell Reports, Volume 1 Supplemental Information Robust Self-Renewal of Rat Embryonic Stem Cells Requires Fine-Tuning of Glycogen Synthase Kinase-3 Inhibition Yaoyao Chen, Kathryn Blair, and Austin
3.1.4 DNA Microarray Technology
 3.1.4 DNA Microarray Technology Scientists have discovered that one of the differences between healthy and cancer is which genes are turned on in each. Scientists can compare the gene expression patterns
3.1.4 DNA Microarray Technology Scientists have discovered that one of the differences between healthy and cancer is which genes are turned on in each. Scientists can compare the gene expression patterns
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Supplemental Information. Metalloprotease-Dependent Onset. of Blood Circulation in Zebrafish
 Current Biology, Volume 20 Supplemental Information Metalloprotease-Dependent Onset of Blood Circulation in Zebrafish Atsuo Iida, Kazuya Sakaguchi, Kiyoaki Sato, Hidetoshi Sakurai, Daigo Nishimura, Aya
Current Biology, Volume 20 Supplemental Information Metalloprotease-Dependent Onset of Blood Circulation in Zebrafish Atsuo Iida, Kazuya Sakaguchi, Kiyoaki Sato, Hidetoshi Sakurai, Daigo Nishimura, Aya
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Sperm cells are passive cargo of the pollen tube in plant fertilization
 In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17079 Sperm cells are passive cargo of the pollen tube in plant fertilization Jun Zhang 1, Qingpei
In the format provided by the authors and unedited. SUPPLEMENTARY INFORMATION VOLUME: 3 ARTICLE NUMBER: 17079 Sperm cells are passive cargo of the pollen tube in plant fertilization Jun Zhang 1, Qingpei
Figure S1. Figure S2. Figure S3 HB Anti-FSP27 (COOH-terminal peptide) Ab. Anti-GST-FSP27(45-127) Ab.
 / 36B4 mrna ratio Figure S1 * 2. 1.6 1.2.8 *.4 control TNFα BRL49653 Figure S2 Su bw AT p iw Anti- (COOH-terminal peptide) Ab Blot : Anti-GST-(45-127) Ab β-actin Figure S3 HB2 HW AT BA T Figure S4 A TAG
/ 36B4 mrna ratio Figure S1 * 2. 1.6 1.2.8 *.4 control TNFα BRL49653 Figure S2 Su bw AT p iw Anti- (COOH-terminal peptide) Ab Blot : Anti-GST-(45-127) Ab β-actin Figure S3 HB2 HW AT BA T Figure S4 A TAG
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of
 Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Supplementary Figure 1. Localization of MST1 in RPE cells. Proliferating or ciliated HA- MST1 expressing RPE cells (see Fig. 5b for establishment of the cell line) were immunostained for HA, acetylated
Single cell resolution in vivo imaging of DNA damage following PARP inhibition. Supplementary Data
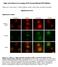 Single cell resolution in vivo imaging of DNA damage following PARP inhibition Katherine S. Yang, Rainer H. Kohler, Matthieu Landon, Randy Giedt, and Ralph Weissleder Supplementary Data Supplementary Figures
Single cell resolution in vivo imaging of DNA damage following PARP inhibition Katherine S. Yang, Rainer H. Kohler, Matthieu Landon, Randy Giedt, and Ralph Weissleder Supplementary Data Supplementary Figures
J. Cell Sci. 128: doi: /jcs : Supplementary Material. Supplemental Figures. Journal of Cell Science Supplementary Material
 Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
No wash 2 Washes 2 Days ** ** IgG-bead phagocytosis (%)
 Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
Supplementary Figures Supplementary Figure 1. No wash 2 Washes 2 Days Tat Control ** ** 2 4 6 8 IgG-bead phagocytosis (%) Supplementary Figure 1. Reversibility of phagocytosis inhibition by Tat. Human
Michal Reichman-Fried
 Zebrafish Development and Genetics course 2013 Cell labeling and migration Imaging primordial germ cell migration Instructors: Erez Raz (erez.raz@uni-muenster.de) Michal Reichman-Fried (mreichm@uni-muenster.de)
Zebrafish Development and Genetics course 2013 Cell labeling and migration Imaging primordial germ cell migration Instructors: Erez Raz (erez.raz@uni-muenster.de) Michal Reichman-Fried (mreichm@uni-muenster.de)
Supplementary methods
 Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
Supplementary methods Cell culture, infection, transfection, and RNA interference HEK293 cells and its derivatives were grown in DMEM supplemented with 10% FBS. Various constructs were introduced into
Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene. Andrew ElBardissi, The Pennsylvania State University
 Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene Andrew ElBardissi, The Pennsylvania State University Abstract: Hong Ma, The Pennsylvania State University The Excess Microsporocytes
Understanding the Cellular Mechanism of the Excess Microsporocytes I (EMSI) Gene Andrew ElBardissi, The Pennsylvania State University Abstract: Hong Ma, The Pennsylvania State University The Excess Microsporocytes
Mitotic cell rounding and epithelial thinning regulate lumen
 Mitotic cell rounding and epithelial thinning regulate lumen growth and shape Esteban Hoijman, Davide Rubbini, Julien Colombelli and Berta Alsina SUPPLEMENTARY FIGURES Supplementary Figure 1 (a-c) Localization
Mitotic cell rounding and epithelial thinning regulate lumen growth and shape Esteban Hoijman, Davide Rubbini, Julien Colombelli and Berta Alsina SUPPLEMENTARY FIGURES Supplementary Figure 1 (a-c) Localization
TALEN mediated targeted editing of GM2/GD2-synthase gene modulates anchorage independent growth by reducing anoikis resistance in mouse tumor cells
 Supplementary Information for TALEN mediated targeted editing of GM2/GD2-synthase gene modulates anchorage independent growth by reducing anoikis resistance in mouse tumor cells Barun Mahata 1, Avisek
Supplementary Information for TALEN mediated targeted editing of GM2/GD2-synthase gene modulates anchorage independent growth by reducing anoikis resistance in mouse tumor cells Barun Mahata 1, Avisek
Stargazin regulates AMPA receptor trafficking through adaptor protein. complexes during long term depression
 Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
Supplementary Information Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long term depression Shinji Matsuda, Wataru Kakegawa, Timotheus Budisantoso, Toshihiro Nomura,
Schematic representation of the endogenous PALB2 locus and gene-disruption constructs
 Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Hong et al.,
 Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
Supplementary Figures Supplementary Figure 1
 Supplementary Figures Supplementary Figure 1 Supplementary Figure 1 COMSOL simulation demonstrating flow characteristics in hydrodynamic trap structures. (a) Flow field within the hydrodynamic traps before
Supplementary Figures Supplementary Figure 1 Supplementary Figure 1 COMSOL simulation demonstrating flow characteristics in hydrodynamic trap structures. (a) Flow field within the hydrodynamic traps before
Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng
 Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Figure S1 Correlation in size of analogous introns in mouse and teleost Piccolo genes. Mouse intron size was plotted against teleost intron size for t
 Figure S1 Correlation in size of analogous introns in mouse and teleost Piccolo genes. Mouse intron size was plotted against teleost intron size for the pcloa genes of zebrafish, green spotted puffer (listed
Figure S1 Correlation in size of analogous introns in mouse and teleost Piccolo genes. Mouse intron size was plotted against teleost intron size for the pcloa genes of zebrafish, green spotted puffer (listed
