Asymmetric localization of the adaptor protein Miranda in neuroblasts is achieved by diffusion and sequential interaction of Myosin II and VI
|
|
|
- Magdalene Beasley
- 6 years ago
- Views:
Transcription
1 Research Article 1403 Asymmetric localization of the adaptor protein Miranda in neuroblasts is achieved by diffusion and sequential interaction of Myosin II and VI Veronika Erben 1, *, Markus Waldhuber 1,2, *, Diana Langer 1, Ingrid Fetka 1, Ralf Peter Jansen 1 and Claudia Petritsch 1,2,3,4, 1 GeneCenter, Ludwig-Maximilian-University Munich, Department of Biochemistry and Laboratory of Molecular Biology, Munich, Germany 2 Department of Neurological Surgery, 3 Brain Tumor Research Center and 4 Helen Diller Comprehensive Cancer Center, University of California, San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143, USA *These authors contributed equally to this work Author for correspondence at present address: University of California, San Francisco (UCSF), Department of Neurological Surgery, 513 Parnassus Avenue, San Francisco, CA , USA ( claudia.petritsch@ucsf.edu) Accepted 5 February 2008 Journal of Cell Science 121, Published by The Company of Biologists 2008 doi: /jcs Summary The adaptor protein Miranda plays a pivotal role in the asymmetric cell division of neuroblasts by asymmetrically segregating key differentiation factors. Miranda localization requires Myosin VI and Myosin II. The apical-then-basal localization pattern of Miranda detected in fixed tissue, and the localization defects in embryos lacking Myosin VI, suggest that Miranda is transported to the basal pole as a Myosin VI cargo. However, the mode and temporal sequence of Miranda localization have not been characterized in live embryos. Furthermore, it is unknown whether Miranda and PON, a second adaptor protein required for asymmetric protein localization, are both regulated by Myosin II. By combining immunofluorescence studies with time-lapse confocal microscopy, we show that Miranda protein forms an apical crescent at interphase, but is ubiquitously localized at prophase in a Myosin-II-dependent manner. FRAP analysis revealed that Miranda protein reaches the basal cortex by passive diffusion throughout the cell, rather than by long-range Myosin VIdirected transport. Myosin VI acts downstream of Myosin II in the same pathway to deliver diffusing Miranda to the basal cortex. PON localization occurs mainly along the cortex and requires Myosin II but not Myosin VI, suggesting that distinct mechanisms are employed to localize different adaptor proteins during asymmetric cell division. Supplementary material available online at Key words: Mechanism for asymmetric cell division, Stem cells, Miranda, PON, Myosin II and VI Introduction Drosophila neuroblasts (NBs) divide in an asymmetric fashion to generate another NB (self-renewal) and a differentiating cell, the ganglion mother cell (GMC) (Lee et al., 2006). To divide asymmetrically, NBs establish an axis of apical/basal polarity, orient the mitotic spindle along this axis, and localize regulators of selfrenewal to the apical pole and key differentiation factors to the basal pole. Upon cytokinesis, the larger, apical daughter cell inherits the self-renewing factors and therefore retains NB fate, whereas the smaller, basal cell inherits differentiation factors and acquires GMC fate (Barros et al., 2003; Yu et al., 2006). Adaptor proteins, such as Miranda and Partner of Numb (PON), play a pivotal role in asymmetric cell division because they ensure the asymmetric segregation of cell fate determinants to the GMC (Betschinger and Knoblich, 2004). Miranda regulates the asymmetric segregation of key differentiation factors such as Brat (a translational repressor) and Prospero (a homeodomain transcriptional repressor) (Ikeshima-Kataoka et al., 1997; Lee et al., 2006; Schuldt et al., 1998; Shen et al., 1997). PON binds to and localizes Numb, a membrane-associated protein and a negative regulator of Notch signaling, to the basal cortex (Lu et al., 1998). In embryos with reduced Myosin VI (Jaguar) activity, Miranda does not form a basal crescent but is mislocalized to the cytoplasm (Petritsch et al., 2003). Myosin VI, unlike all other characterized myosins, moves processively towards the minus end of actin filaments, taking large steps, but can also function as an actin-based anchor (Sweeney and Houdusse, 2007). Myosin VI protein is abundantly expressed in NBs, where it transiently accumulates in the basal half of metaphase NBs and partially co-localizes with Miranda (Petritsch et al., 2003). Myosin VI forms a complex with Miranda and Prospero in Drosophila embryonic extracts and shows direct physical interactions with Miranda in vitro (Petritsch et al., 2003). These observations suggested that Miranda might be transported from the apical to the basal cortex as a Myosin VI cargo. Miranda also shows a physical interaction with Zipper, the heavy chain of Myosin II (Petritsch et al., 2003). Myosin II is a plus enddirected motor that forms bipolar filaments as a heterohexamer (Bresnick, 1999). Earlier data have suggested that Zipper antagonizes basal crescent formation by negatively interacting with Lethal giant larvae [Lgl; L(2)gl] (Ohshiro et al., 2000; Peng et al., 2000). The zygotic zipper mutant has intact asymmetric cell division most likely due to maternal contribution of wild-type Zipper. More recently, it has been shown that Myosin II is activated through phosphorylation by Rho kinase, and can be selectively inhibited by a Rho kinase inhibitor (Barros et al., 2003). Myosin II localizes asymmetrically to the apical pole at prophase and moves
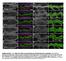
2 1404 Journal of Cell Science 121 (9) to the basal side along the cortex, accumulating at the cleavage furrow (Barros et al., 2003; Strand et al., 1994). As inhibition of Myosin II caused Miranda to mislocalize uniformly around the cortex, and because Myosin II and Miranda localize almost exclusively, it has been proposed that active Myosin II filaments on the apical pole exclude Miranda from the cortex, rather than transport Miranda from the apical to the basal cortex (Barros et al., 2003). Myosin II and Myosin VI both interact with the tumor suppressor Lgl to localize Miranda. Myosin VI acts synergistically to Lgl to properly localize Miranda (Petritsch et al., 2003). Only after Lgl is phosphorylated and inactivated by Drosophila atypical protein kinase C (apkc) at the apical cortex (Betschinger et al., 2003) can Myosin II become activated (Barros et al., 2003). apkc is part of the conserved Par complex consisting of Bazooka (Baz; also known as Par-3), Par-6 and apkc, which, together with a second apical complex comprising of Inscuteable, Partner of Inscuteable (Pins; Rapsynoid FlyBase) and G protein αi, regulates the basal localization of Miranda and PON and their binding partners (Yu et al., 2006). Immunofluorescence staining on fixed tissue detected Miranda in an apical crescent, as well as in the cytoplasm, prior to the formation of a basal metaphase crescent (Barros et al., 2003; Fuerstenberg et al., 1998; Ikeshima-Kataoka et al., 1997; Petritsch et al., 2003; Shen et al., 1997). These data suggest a dynamic, stepwise pattern for Miranda localization, but the mode and temporal sequence of Miranda localization has not yet been studied at precisely defined stages during NB mitoses in live embryos. Furthermore, it is not known at what stage of Miranda localization Myosin II and Myosin VI act, and whether they cooperate in the same pathway to localize Miranda. As previously determined by time-lapse confocal microscopy, PON protein is recruited from the cytoplasm to the cortex at interphase in NBs, and moves two-dimensionally along the cortex to a basal cortical crescent (Lu et al., 1999). FRAP analysis of PON in sensory organ precursor cells of Drosophila pupae suggested that PON becomes rapidly recruited from juxta-cortical areas to form a basal cortical crescent by binding to a high-affinity binding partner. PON localization depends on apkc activity and the phosphorylation status of Lgl (Mayer et al., 2005), and is sensitive to 2,3-butanedione monoxime (BDM), an inhibitor of myosin ATPase activity (Lu et al., 1999). These data suggest that PON, like Miranda, requires Myosin motor activity for basal localization. As Miranda and PON both localize to a basal crescent in metaphase in a myosindependent fashion, it is possible that they engage similar molecules, such as the cortical Myosin II, to reach their destination. A requirement of Myosin II for PON localization has not yet been studied. To address these open questions, we performed first a quantitative analysis of Miranda localization using markers to define individual stages of NB mitosis. Thorough quantitative analyses showed that Miranda localized predominantly to an apical crescent at interphase but was rather ubiquitously localized, with a strong cytoplasmic component at prophase. Miranda mrna remained apically localized during NB mitosis, and short-term inhibition of proteasome-dependent degradation still allowed for the formation of a metaphase crescent, suggesting that Miranda protein forms a basal crescent by protein movement rather than by localized translation and/or degradation. Time-lapse confocal microscopy on embryos expressing Miranda-GFP in combination with FRAP revealed that cytoplasmic Miranda protein moved rapidly by passive diffusion rather than by myosin-directed transport. Cytoplasmic Miranda localization at pro/metaphase required active Myosin II. Myosin VI is required at a subsequent step to bring ubiquitously diffusing Miranda to the basal crescent at metaphase. By simultaneously inhibiting Myosin II and Myosin VI, we showed that Myosin II acts upstream of Myosin VI in the same pathway to localize Miranda. Asymmetric localization of PON required Myosin II but not Myosin VI, suggesting differences in the localization machinery of the two adaptor proteins Results Miranda protein localizes ubiquitously to the cytoplasm and cortex at prophase, and forms a basal crescent independent of basal translation or protein degradation Previous data investigating Miranda localization in fixed embryonic tissue detected Miranda protein in an apical cortical crescent prior to formation of a basal crescent, leading to the hypothesis that Miranda receives a signal at the apical pole instructing it to localize to a basal crescent (Fuerstenberg et al., 1998; Ikeshima-Kataoka et al., 1997; Matsuzaki et al., 1998; Shen et al., 1998). The exact timing of the formation of the apical Miranda crescent remained controversial. Several reports stated that Miranda is apical at interphase and/or at prophase (Fuerstenberg et al., 1998; Matsuzaki et al., 1998; Shen et al., 1998), although one report showed that Miranda localizes to the cytoplasm at interphase (Ikeshima-Kataoka et al., 1997). In these earlier studies, DNA condensation was used to distinguish between interphase and prophase. To address the controversy about the timing of apical localization during the NB cell cycle, and to better correlate the distinct patterns of Miranda localization with well-defined stages of the cell cycle, we stained fixed embryonic tissue for Miranda, γ-tubulin (to mark the centrosome) and apkc (to mark the apical crescent). Centrosomes duplicate on either the apical or basal side of the cell, and migrate laterally to become positioned at opposite poles along the apical/basal axis at pro/metaphase (Kaltschmidt et al., 2000). At early and later stages of prophase, when centrosomes were migrating laterally, Miranda localized mainly to the cytoplasm and the cortex, but not to the nucleus (Fig. 1A). At pro/metaphase, when centrosomes moved towards opposite poles and the nuclear membrane breaks down, Miranda filled the entire cytoplasm, including the nuclear regions. At metaphase, Miranda disappeared from the cytoplasm and formed a basal crescent, which was segregated exclusively to the GMC at telophase (Fig. 1A). As previously shown, apkc remained apical during mitosis and was downregulated in telophase. Next, we determined the frequency of the apical Miranda crescent, and also of cytoplasmic and cortical Miranda, during interphase and compared it to that at prophase (Fig. 1B-D). At interphase, Miranda protein was localized to an apical crescent in the majority of NBs (75.7±1.4%); only in a small proportion of NBs was Miranda apically enriched (5.4±1.2%), localized to the cytoplasm and the cortex (12.4±3.8%), or localized to the cytoplasm only (6.6±3.7%; n=255; Fig. 1B,C). At prophase, apical localization of Miranda was rare (apical crescents, 3.7±1.4%; apically enriched, 3.7±3.1%). In the majority of NBs, Miranda was localized to the cytoplasm and the cortex (prophase/1, 76.1±6.9%) or to the cytoplasm only (prophase/2, 16.5±5.3%; n=193; Fig. 1B,D). Taken together, we found that Miranda was predominantly localized to an apical crescent during interphase, but rarely at prophase. Our data suggest that apical localization of Miranda is very transient at prophase, and disappears early at the onset of
3 Dynamics of Miranda localization 1405 Fig. 1. (A) Miranda protein localization at defined steps during NB mitosis. Apical is up in all figures. Bars, 5 μm. At early prophase and prophase, centrosomes move laterally, apkc accumulates at the apical cortex, and Miranda protein predominantly localizes to the cytoplasm and the cortex. At pro/metaphase, centrosomes are positioned at opposite poles along the apical/basal axis, apkc is apical and Miranda protein fills the entire cell, including the nuclear region. At metaphase, centrosomes remain aligned along the apical/basal axis, apkc is apical and Miranda is localized entirely to a basal cortical crescent. At telophase, the apkc signal is weak in the NBs, and Miranda is exclusively inherited by the GMC. Miranda, red; PKC and γ-tubulin, green; DNA, blue. (B) Miranda forms an apical crescent at interphase. At interphase, the centrosomes have not yet duplicated and Miranda predominantly forms an apical crescent overlaying the centrosome. At prophase, Miranda is predominantly cytoplasmic with (Prophase 1) or without (Prophase 2) a cortical component. (C,D) Quantification of different localization stages of Miranda at interphase and prophase. (C) At interphase, Miranda protein is localized to an apical crescent in the majority of NBs, and is apically enriched; cytoplasmic and cortical localization, and cytoplasmic only localization is observed in only a fraction of NBs. (D) At prophase, apical localization of Miranda is rare. The majority of NBs show cytoplasmic and cortical, or only cytoplasmic Miranda. (E) miranda mrna remains apically localized throughout NB mitosis. miranda mrna is apically enriched at prophase and partially co-localizes with Miranda protein (white arrowhead). At metaphase, miranda mrna (white brackets) remains apical, whereas Miranda protein is exclusively localized in the basal cortical cresent (white arrowhead). At anaphase and telophase, miranda mrna remains in the NBs, whereas Miranda protein is found in the GMC. No signal for miranda mrna can be detected using a sense RNA probe as a control (Metaphase control ). miranda mrna, green; Miranda protein, red; DNA, blue. The NB at telophase is marked by a white circle. (F-I) Inhibition of the proteasome prevents cyclin A degradation at metaphase and progression to anaphase, but does not affect Miranda localization. Miranda protein still forms a basal metaphase crescent in NBs of embryos treated with DMSO as control (F) or MG132 (G). Cyclin A is degraded in the majority of metaphase NBs of control embryos (F) but persists in metaphase NBs of MG132-treated embryos (G). Quantification of metaphase versus ana/telophase NBs (H), and Miranda metaphase crescents versus metaphase with persistent cyclin A (I), reveals that 30 minutes, but not 15 minutes, with MG132 inhibited the progression of metaphase NBs to anaphase and the efficient degradation of cyclin A. Miranda protein is localized to a basal crescent in the majority of MG132-treated NBs.
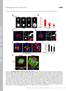
4 1406 Journal of Cell Science 121 (9) mitosis of NBs to give rise to the ubiquitous localization of Miranda at prophase and pro/metaphase. Miranda localization could be explained by dynamic protein movement, or, alternatively, by localized translation of Miranda protein at the basal pole and its localized degradation at the apical pole at metaphase. To investigate whether miranda mrna localized to a basal crescent and overlapped with Miranda protein at metaphase, we performed fluorescent in situ hybridization and visualized Miranda protein by immunohistochemistry (Fig. 1C; n=30). In agreement with earlier data that characterized prophase NBs, miranda mrna accumulated around the apical pole and partially colocalized with cytoplasmic Miranda protein at prophase (Schuldt et al., 1998) (Fig. 1E). Although miranda mrna remained apical at meta- and anaphase, and exclusively segregated to the NB daughter, Miranda protein localized to a basal metaphase crescent and segregated to the GMC (Fig. 1E). Thus, Miranda protein and miranda mrna localize exclusively at the time when Miranda protein becomes basally localized. Four potential destruction boxes, which are known to target proteins for proteasome-dependent degradation, reside in the central and C-terminal domain of Miranda (Shen et al., 1997). This suggests that the Miranda protein could be locally degraded by the 26S proteasome in areas outside of the basal metaphase crescent. To investigate a potential role of 26S proteasome-dependent degradation for basal Miranda localization, we treated embryos with the proteasome inhibitor MG132 for 15 or 30 minutes, and detected Miranda protein by immunohistochemistry (Muro et al., 2002). 100% and 98.5% (s.d. ±2.1) of metaphase NBs showed normal, basal localization of Miranda protein after MG132-treatment for 15 minutes (n=167) and 30 minutes (n=153), respectively (Fig. 1G,I). 26S proteasome degrades cyclin A during metaphase (Tio et al., 2001), and, in the absence of proteasome activity, cyclin A persists and cells arrest at metaphase (Sigrist et al., 1995). To control for successful proteasome inhibition, we detected cyclin A in addition to Miranda (Fig. 1F,G,I) and determined the ratio of NBs at metaphase versus ana-/telophases (Fig. 1H). After 30 minutes of MG132-treatment, cyclin A protein persisted in 75.3% (s.d. ±11.5) of metaphase NBs (Fig. 1F,G) (n=153). In untreated embryos, cyclin A was detected in only 29% (s.d. ±5.3; n=205), and in control embryos in only 34% (s.d. ±1.8; n=178), of metaphase NBs (Fig. 1F,I). Coinciding with defective cyclin A degradation, the number of metaphase NBs increased to 84.6% (s.d. ±5.7) after 30 minutes with MG132, from 51% (s.d. ±10) in untreated and from 51.8% (s.d. ±7.8) in control embryos (Fig. 1H). Cyclin A was still properly degraded after 15 minutes with MG132 (28.3±4.7%; n=167) or DMSO treatment (30.4±6.5%; n=119), and the number of metaphase NBs was not significantly altered (MG132-treated, 47.9±4.4% compared with control embryos, 42.7±11.5%). Our data showed that short-term inhibition of proteasome activity did not disrupt the basal localization of Miranda protein. In summary, we conclude that Miranda localization depends on the dynamic movement of a pre-existing pool of Miranda protein from the apical cortex throughout the entire cell to the basal cortex, rather than on the localized translation of miranda mrna at the basal cortex, or on localized Miranda protein degradation at areas outside the basal cortex. Miranda is ubiquitously localized and accumulates in the cytoplasm prior to formation of a basal crescent Immunohistochemistry on fixed tissue does not distinguish between an active, myosin-directed movement and a passive diffusion of Miranda protein. To study the dynamics of Miranda protein localization, we have generated transgenic Drosophila embryos carrying full-length UAS-Miranda-GFP. Different Gal4- driver strains were used to express Miranda-GFP and to follow its localization in NBs and neuroepithelial (NE) cells by timelapse confocal microscopy. As expected, Miranda protein accumulated at the apical pole in only a small proportion of prophase NBs (Fig. 2A). In the majority of NBs and in all NE cells, Miranda localized uniformly to the cytoplasm and the cortex, but not to the nucleus, at early prophase (see Fig. 2A,B). Miranda accumulated in patches throughout the entire cytoplasm, including in the nuclear region during nuclear envelope breakdown, at pro/metaphase and gradually accumulated in a basal crescent at metaphase. As expected, the basal Miranda crescent is symmetrically distributed between the two daughter cells of NE fate upon cytokinesis (Fig. 2B). Miranda is inherited entirely by the GMC in NB divisions (Fig. 2A; see Movies 1 and 3, supplementary material). To ensure that the ubiquitous localization of Miranda was not caused by artificial saturation of the localization machinery as a result of the ectopic expression of Miranda-GFP, we used Gal4- driver strains of different strengths. Miranda-GFP expressed under control of neuralized-gal4 (Fig. 2A,B), a strong NB- and NEspecific driver, V32A-Gal4 (Fig. 2C), driving maternal gene expression, or scabrous-gal4 (Fig. 2D), a weaker NE- and NBspecific driver, showed very similar protein localization patterns, including strong cytoplasmic localization of Miranda at pro/metaphase. As an additional control, we investigated the localization of Miranda-GFP and total Miranda protein by immunohistochemistry (Fig. 2E,G,H). The pattern of Miranda-GFP localization from two lines generated by our lab (Fig. 2G,H) was compared with a preexisting line (Fig. 2E) (Ohshiro et al., 2000). All three lines showed overlapping localization of Miranda-GFP with total Miranda (Fig. 2E, data not shown), and at metaphase (Fig. 2E,G,H). In line 1, a very small fraction of Miranda-GFP localized to the mitotic spindle (Fig. 2E); however, spindle association was not observed in line 2 (Fig. 2G) or line 3 (Fig. 2H). All three lines were used interchangeably in live imaging experiments and showed very similar Miranda-GFP localization patterns although the signal intensity varied. Furthermore, immunoblotting showed that Miranda-GFP was expressed at lower levels than endogenous Miranda protein (Fig. 2I). In embryos expressing Miranda-GFP under Gal4-control, but not in embryos carrying only the Miranda-GFP transgene or just the Gal4 driver, a single band of 130 kda was recognized by the GFP antibody. In addition, endogenous Miranda was recognized as a kda duplet by the Miranda antibody. Taken together, our data suggest that Miranda-GFP does not saturate the localization machinery but rather faithfully recapitulates the localization of endogenous Miranda protein in live embryos. Finally, we evaluated the behavior of Miranda-GFP compared with endogenous Miranda, by studying Miranda-GFP localization in embryos expressing a constitutively active, nonphosphorylatable form of Lgl (Lgl 3A ). Lgl 3A disrupts Miranda localization (Betschinger et al., 2003), presumably by preventing the activation of Myosin II (Barros et al., 2003). Intriguingly, cytoplasmic Miranda was completely abolished when coexpressed with Lgl 3A, suggesting that Lgl is required for the cytoplasmic localization of Miranda at pro/metaphase. Instead, Miranda-GFP, similar to endogenous Miranda (Betschinger et al., 2003), accumulated uniformly around the cortex
5 Dynamics of Miranda localization 1407 Fig. 2. Miranda localization is a dynamic, multistep process in NBs and NE cells. Dynamic localization of Miranda detected by live imaging corresponds to its localization in fixed tissue, as detected by immunohistochemistry. Single confocal sections are shown. Embryos expressing Miranda-GFP under control of neuralized-gal4 (neura-gal4) were examined by time-lapse confocal microscopy for neuroblasts (NB) or neuroepithelial (NE) cells undergoing mitosis. In the majority of NBs (A) and in all NE cells (B), Miranda-GFP localizes uniformly to the cytoplasm and the cortex, but not to an apical crescent at prophase. At pro/metaphase cytoplasmic Miranda-GFP is more intense (white arrowhead), and includes nuclear and cortical areas. At metaphase, the basal cortical crescent is formed and Miranda-GFP gradually disappears from the remaining areas of the cell. Miranda-GFP is inherited by the GMC at telophase in NBs (A; white circle), and by both NE cells in a symmetric division (B). Miranda-GFP shows a very similar cytoplasm-to-basal cortex localization pattern when expressed under the control of V32-Gal4 (C) and scabrous-gal4 (sca-gal4) (D). Cytoplasmic Miranda accumulation is indicated by white arrows. (E) Miranda-GFP recapitulates the localization pattern of total Miranda protein. In fixed embryos, the location of Miranda-GFP is indistinguishable from that of total Miranda, in the cytoplasm at prophase and at the basal crescent at metaphase. Miranda, red; GFP, green; DNA, blue. (F) In embryos expressing an unphosphorylatable form of Lgl, UAS-Lgl 3A, Miranda-GFP is found uniformly around the cortex and cytoplasmic localization is abolished. (G,H) Miranda-GFP localizes to a tight metaphase crescent overlapping with total Miranda in two additional transgenic lines (line 2, G; line 3, H). (I) Immunoblotting using a Miranda antibody (top panel) and a GFP antibody (middle panel) reveals that ectopically expressed Miranda-GFP represented by the 130 kda band is specifically expressed in UAS-Miranda- GFP/scabrous-Gal4 embryos, but not in UAS-Miranda-GFP or scabrous-gal4 embryos (controls). Miranda-GFP levels are low compared with total Miranda protein, which runs at kda. Tubulin was detected as a control for equal loading (bottom panel). at prophase and at metaphase, and symmetrically segregated at anaphase (Fig. 2F). Taken together, we conclude that Miranda, after very transiently forming an apical crescent, becomes localized uniformly to the cytoplasm and the cortex at prophase, and ubiquitously throughout the cell during pro/metaphase. At metaphase, Miranda protein gradually forms a basal crescent and disappears from the remainder of the cell.
6 1408 Journal of Cell Science 121 (9) PON protein localization requires Myosin II but not Myosin VI Previous data show that the asymmetric localization of PON is sensitive to butanedione-2-monoxime (BDM), a well-studied inhibitor of the ATPase of skeletal muscle Myosin II. BDM efficacy towards other, non-muscle myosins remains controversial (Ostap, 2002), and thus it is not known yet which myosin motors regulate PON localization. To address a potential role of Myosin II and Myosin VI in PON localization, we repeated time-lapse confocal imaging of PON-GFP in dividing NBs and NE cells using a preexisting transgenic Drosophila strain (Fig. 3) (Lu et al., 1999). As shown earlier, PON was cleared from the cytoplasm at interphase to localize mainly uniformly around the cortex at prophase (data not shown) (Lu et al., 1999). At pro/metaphase, PON protein moved basolaterally mainly along the cortex, and gradually accumulated in a basal metaphase crescent in NBs (Fig. 3B) and NE cells (Fig. 3D, see also Movies 2 and 4 in the supplementary material). PON lacked the strong cytoplasmic localization (Lu et al., 1999) of Miranda protein at prophase and pro/metaphase in NBs (Fig. 3A) and NE cells (Fig. 3C), suggesting that the two adaptor proteins are using distinct routes to localize to a metaphase crescent. To test whether the spatial differences in PON and Miranda localization at pro/metaphase are reflected in mechanistic differences, we investigated the role of Myosin II and VI for PON localization. We inhibited Myosin II indirectly by injection of the Rho kinase inhibitor Y into PON-GFP-expressing embryos and then examined the embryos by live imaging (Fig. 3F). In the absence of fully functional Myosin II, PON was mislocalized around the cortex at pro- and metaphase. NBs in control-injected embryos underwent cytokinesis and PON was segregated to the GMC (Fig. 3E), NBs in Myosin II-inhibited embryos, however, showed abnormal division and PON accumulated at the cleavage furrow (Fig. 3F). Thus, we propose that PON is excluded from the apical cortex at prophase and moved to a basal crescent at pro/metaphase by Myosin II. Taken together with earlier data showing that Myosin Fig. 3. PON takes a different route to the basal crescent than Miranda, and requires Myosin II but not Myosin VI. Time-lapse analysis to compare the localization of Miranda-GFP (A,C) with PON-GFP (B,D) shows that PON localizes mainly along the cortex at pro/metaphase in NBs (B), as well as in NE cells (D). PON-GFP does not accumulate in the cytoplasm (white arrowheads). Miranda-GFP consistently showed strong cytoplasmic localization in NBs (A), as well as in NE cells (C, white arrowheads). At metaphase, Miranda (A,C) and PON (B,D) form an overlapping basal crescent. (E,F) Time-lapse microscopy of PON-GFP localization in NBs from untreated, control embryos (E), or NBs from embryos lacking fully functional Myosin II (F) because of injection of a Rho kinase inhibitor. In the absence of Myosin II activity, PON-GFP does not form a basal crescent but is mislocalized to the cortex in metaphase and anaphase, and concentrates at the cleavage furrow in telophase (arrowheads). (G) Downregulation of Myosin VI by RNA interference (MyoVI RNAi) does not affect crescent formation at metaphase, or the asymmetric segregation of PON-GFP at ana- and telophase. The mitotic spindle and, hence, the cleavage plane are rotated by 45 owing to downregulation of Myosin VI. The white circle in telophase depicts the position of the NB. Embryos heterozygous (H) and homozygous (I) for the jar 1 allele were fixed and stained with a PON antibody (green). DNA, blue. In agreement with the live imaging data, PON still formed a basal crescent at metaphase in control (H) and mutant (I) embryos. The mitotic spindle is misoriented by 90 owing to the lack of Myosin VI activity.
7 Dynamics of Miranda localization 1409 II localizes to the cleavage furrow at telophase (Barros et al., 2003), PON might be pushed into the GMC at telophase by Myosin II activity. The pointed end-directed Myosin VI is required for basal localization of Miranda (Petritsch et al., 2003). Myosin VI predominantly localizes to patches throughout the cytoplasm and the cortex in prophase (Petritsch et al., 2003), which suggests that it does not affect PON localization by directly binding to PON. To test for a potential indirect function of Myosin VI in PON localization, we injected Myosin VI double-stranded RNA in embryos expressing PON-GFP to downregulate Myosin VI activity (MyoVI RNAi, Fig. 3G) (Petritsch et al., 2003). Previous data showed that the mitotic spindle is misoriented in embryos lacking fully functional Myosin VI (Petritsch et al., 2003) (Fig. 3G,H). Time-lapse confocal imaging revealed that PON-GFP protein still formed a basal cortical crescent in metaphase and was segregated exclusively to the GMC. Because of the misorientation of the mitotic spindle, the division plane was rotated by 45 and the PON crescent was now positioned laterally to the epithelial surface (Fig. 3G). In an alternative approach, we studied PON localization by immunohistochemistry on a zygotic mutant allele of Myosin VI, jaguar (jar 1 ) (Petritsch et al., 2003). PON localization was normal in metaphase NBs of embryos heterozygous mutant for jar 1 with normal spindle orientation (Fig. 3H) and homozygous mutant for jar 1 with misoriented mitotic spindle (Fig. 3I). We concluded that, in contrast to its function for Miranda localization, Myosin VI is dispensable for the asymmetric localization of PON in a cortical metaphase crescent and its segregation to the GMC. Distinct modes of cytoplasmic and cortical Miranda protein movement To distinguish between a Myosin VI-directed movement and passive diffusion of Miranda at pro/metaphase (Petritsch et al., 2003), we determined fluorescence recovery after photobleaching (FRAP) of Miranda-GFP. The chromosomal condensation state was visualized by co-expressing Histone2A (His2AvD-mRFP) (Schuh et al., 2007) to pinpoint the mitotic stages of dividing NBs. To investigate FRAP of cytoplasmic Miranda, we selectively bleached various regions of interest (ROIs) covering cytoplasmic Miranda at pro/metaphase in dividing NBs (Fig. 4A,B,D). We attempted to calculate the motility of cytoplasmic Miranda-GFP by recording the recovery of the fluorescent signal by Miranda- GFP molecules moving into the ROIs from adjacent areas (see Materials and Methods). However, we could not significantly reduce the fluorescent signal when bleaching a cytoplasmic ROI on either the apical (Fig. 4A) or the basal (Fig. 4B) half of the cell by applying the same parameters, which significantly reduced the cortical Miranda-GFP signal that was used as a reference (Fig. 4E). These results were indicative of a rapid movement of Miranda molecules with a t 1/2 of less than 1.5 seconds, and suggested that Miranda diffused unrestrictedly throughout the cytoplasm. As a control for a freely diffusing protein, we studied FRAP of egfp, which showed a very similar recovery rate, with a t 1/2 of less than one second (t 1/2 <1 second; Fig. 4C; data not shown). The recovery rate of Miranda-GFP was slightly slower than that of egfp alone, which could be explained by the greater molecular mass of the fusion protein and by its association with other diffusible molecules or cargo molecules (Fig. 4C). We conclude that cytoplasmic Miranda moves freely within the cytoplasm, with a high mobility suggestive of passive diffusion rather than active transport. Next, we tested whether Miranda protein at pro/metaphase is required for the formation of a basal crescent at metaphase, by selectively bleaching the entire NB at pro-metaphase. No basal Miranda-GFP crescent was recovered after bleaching cytoplasmic Miranda (Fig. 4D). This study corroborated our earlier data showing that a preexisting pool of Miranda protein at pro/metaphase is required for proper formation of the basal metaphase crescent. Miranda could form a cortical crescent by attaching to a cortical anchor, which restricts protein movement within basal areas. To test this hypothesis, we analyzed FRAP of Miranda-GFP in the basal crescent (Fig. 4E) and compared it to its mobility in the cytoplasm (Fig. 4F) and to the cortical mobility of PON (Fig. 4G). Indeed, FRAP of Miranda-GFP at the basal cortex took significantly longer than did the recovery of cytoplasmic Miranda-GFP (Fig. 4F). Our data suggested that cortical Miranda has a lower mobility and might thus be anchored at the basal cortex. A comparison of the FRAP kinetics of cortical PON and Miranda revealed that their half-time recovery values were indeed very similar (t 1/2 Miranda, 6.76±0.66 seconds; t 1/2 PON, 6.78±0.43 seconds; Fig. 4G,H). By removing the basal Miranda crescent at either edge, we determined that Miranda fills either area at a comparable speed, but does not fill areas outside the pre-existing basal crescent. In NBs, PON-GFP also retained mobility only within the limits of the basal crescent (Lu et al., 1999). These data suggest that Miranda and PON are confined to the limits of a basal cortical crescent by attaching to perhaps the same, as yet unidentified, cortical anchor (Mayer et al., 2005). Myosin II and Myosin VI act at distinctive steps in the same pathway to localize Miranda Previous experiments analyzing the role of Myosin II and Myosin VI in Miranda localization captured only a static image of defects in mutant embryos (Barros et al., 2003; Peng et al., 2000; Petritsch et al., 2003). In the absence of Myosin II activity, Miranda is mislocalized uniformly around the cortex (Barros et al., 2003). Thus, Myosin II could be required for either formation or, alternatively, maintenance of the basal crescent. To distinguish between these two possibilities, we injected embryos expressing Miranda-GFP and Histone-RFP with the Rho kinase inhibitor Y at a concentration that did not fully inhibit cytokinesis but that downregulated Myosin II activity. This approach allowed us to follow Miranda localization in the absence of Myosin II activity throughout mitosis (Fig. 5B), and to compare it with control embryos (Fig. 5A). Cytoplasmic localization of Miranda, the formation of a basal crescent and the asymmetric segregation of Miranda were completely inhibited by injection of the Rho kinase inhibitor (Fig. 5B). These results suggest that Myosin II is required at prophase to allow Miranda to move to the cytoplasm. To study the function of Myosin VI at defined stages of Miranda localization, we downregulated Myosin VI activity by injecting live embryos expressing Miranda-GFP with myosin VI dsrna (Petritsch et al., 2003) (Fig. 5C). In the absence of fully functional Myosin VI, Miranda-GFP accumulated in patches thoughout the entire cell; it did not form a basal crescent and thus segregated symmetrically to both daughter cells (Fig. 5C). Myosin VI itself partially colocalizes with Miranda, mainly in the cytoplasm (Petritsch et al., 2003). Thus, we propose that Myosin VI is required at pro/metaphase to bring cytoplasmically localized Miranda to the basal cortex.
8 1410 Journal of Cell Science 121 (9) Fig. 4. Miranda moves three-dimensionally in the cytoplasm by passive diffusion, but shows a spatially limited and slower movement at the cortex. (A-E) FRAP experiments in living embryos co-expressing Miranda-GFP (green) and Histone2A-mRFP (red) or PON-GFP. White circles indicate selectively bleached regions. (A,B) Prebleach and postbleach images of prophase NBs are shown. Cytoplasmic Miranda-GFP could not be bleached on the apical (A) or basal (B) side of the cell by using the same bleaching intensity that was used to eliminate signal from cortical Miranda-GFP (E), indicative of three-dimensional diffusion. (C) Cytoplasmic Miranda-GFP and freely diffusing egfp showed similar kinetics. Both have a significantly shorter recovery time than Miranda at the basal cortex (E). (D) Miranda-GFP at prophase and pro/metaphase. Miranda-GFP was repeatedly bleached at high laser intensity at pro/metaphase to remove signal from the NB (white circle). Subsequently, no basal crescent was detected in metaphase (white brackets), suggesting that ubiquitously localized Miranda at pro/metaphase is required to form the basal crescent at metaphase. (E) Miranda-GFP signal bleached at the basal cortical crescent (white circle) recovered at a slower rate than did cytoplasmic Miranda, suggesting that Miranda does not diffuse freely at the cortex. (F) Quantification of the relative fluorescent intensity over time shows that cortical Miranda moves slower (t 1/2 =6.76±0.67 seconds) than cytoplasmic Miranda (t 1/2 <1.5 seconds), but at similar rate to cortical PON- GFP (t 1/2 =6.78±0.43 seconds). The recovery rates of Miranda and PON at the cortex are still fast, indicating that the two proteins show dynamic association with the cortex. Myosin II and Myosin VI may act either sequentially in the same pathway or in parallel pathways at distinct steps to localize Miranda. Analyzing a potential interaction of Myosin II and Myosin VI has been hampered by the overall abnormal morphology of the double mutant for the Myosin VI and the Myosin II heavy chain (Petritsch et al., 2003). As an alternative approach, we injected both the Rho kinase inhibitor (to inhibit Myosin II) and myosin VI dsrna into live embryos and followed Miranda movement by time-lapse confocal microscopy. In the double knockdown NBs, cytoplasmic localization of Miranda was eliminated and Miranda localized uniformly around the cortex from meta- to telophase (Fig. 5D). Miranda mislocalization in the absence of both myosins closely resembled the pattern of mislocalization seen with reduced Myosin II activity alone (Fig.
9 Dynamics of Miranda localization 1411 Fig. 5. Myosin II and Myosin VI act at distinctive steps in the same pathway to localize Miranda. Live imaging of embryos co-expressing Miranda-GFP (green) and Histone2A-mRFP (red) injected with buffer as a control (A), a Rho kinase inhibitor to downregulate Myosin II (B), treated by MyoVI RNAi (C), or both RKI and MyoVI RNAi (D). After injections, embryos were fixed and stained with anti-miranda antibody (green). DNA, red. 5B), rather than an additive or a Myosin VI loss-of-function phenotype (Fig. 5C). Taken together, we propose that Myosin II activity mobilizes Miranda at the apical cortex at early prophase. Miranda moves to the cytoplasm and diffuses throughout the cell at pro/metaphase. Myosin VI transitions Miranda from the cytoplasm to the basal cortex at metaphase. Myosin II and Myosin VI act sequentially in the same pathway to localize Miranda. Discussion Miranda is asymmetrically localized by protein movement Here, we show that the dynamic localization of Miranda is achieved primarily by protein movement rather than by alternative mechanisms, such as the localized translation of miranda mrna at the basal cortex and localized degradation at areas outside of the basal cortex. miranda mrna localized exclusively to Miranda protein at metaphase (Fig. 1E), and although we cannot exclude that small amounts of mrna, undetectable by our experimental approach, are translated at the basal cortex, we propose that they do not significantly contribute to Miranda protein localization. To test for localized degradation of Miranda protein, we initially quantified Miranda localization in embryos carrying a dominant temperature-sensitive (DTS) proteasome mutation in the β2 proteasome subunit gene DTS5 (Pros26), or in embryos expressing a copy of the DTS5 mutant in NBs (Schweisguth, 1999). However, cyclin A degradation, and progression from metaphase to anaphase, is not strongly affected in this mutant at the restrictive temperature (Tokumoto et al., 1997) (data not shown), and thus we turned to chemical inhibition of the proteasome by short-term treatment with MG132. In a recent study, MARCM clones for Tbp-1, a gene encoding a regulatory subunit of the proteasome, show mislocalization of Miranda in larval NBs, suggesting that Miranda localization is affected by long-term inhibition of the proteasome. However, because Miranda protein is only mono- and not poly-ubiquitylated, which is a prerequisite for proteasome-dependent degradation, Slack et al. proposed that the proteasome affects Miranda protein localization indirectly (Slack et al., 2007). This interpretation is in line with our data showing that short-term inhibition with MG132 efficiently inhibits the proteasome, leading to the persistence of cyclin A and metaphase arrest, but does not affect Miranda protein localization (Fig. 1F-I). Diffusion of Miranda protein throughout the cell precedes basal crescent formation Our experiments reveal that Miranda localizes to an apical crescent at interphase but is uniformly localized to the cytoplasm and the cortex at prophase, and accumulates in the cytoplasm at pro/metaphase (Fig. 2). We performed several control experiments to exclude that the ubiquitous localization and cytoplasmic accumulation of Miranda is caused by saturation of the localization machinery (Fig. 2). Miranda-GFP also functionally rescues Prospero
10 1412 Journal of Cell Science 121 (9) localization in a zygotic Miranda loss-of-function mutant (Peng et al., 2000) (data not shown). In embryonic and larval NBs, a small proportion of Miranda localizes around the centrosome at the pole opposite the crescent formation (Petritsch et al., 2003) (Fig. 1A). A short isoform of Miranda is a component of the centrosome and is localized dynamically to the mitotic spindle in syncytial Drosophila embryos (Mollinari et al., 2002). It is feasible that a fraction of the cytoplasmic pool of Miranda at prophase associates around the apical centrosome and moves to nuclear areas during nuclear envelope breakdown, but, because of the lack of an additional centrosome marker in our analysis, we cannot make this conclusion. We could show, however, that the cytoplasmic Miranda localization does not depend on an intact microtubule network, as we could still detect cytoplasmic Miranda in embryos injected with colcemid to disrupt the mitotic spindle (data not shown). The cytoplasmic localization of Miranda in NBs could have a general relevance. In C. elegans, the conserved Par proteins direct a polarized cytoplasmic flow to move P granules to the posterior cortex of the zygote (Cheeks et al., 2004). Thus, similar to C. elegans zygotes, Drosophila NBs employ a Par protein-dependent cytoplasmic movement to drive the Miranda complex to the basal pole. Adaptor proteins take different routes to the basal cortex but require Myosin II In agreement with earlier data, we found that PON moves mainly two-dimensionally along the cortex to become restricted to a basal metaphase crescent in embryonic NBs (Fig. 3B) (Lu et al., 1999). PON does not accumulate in the cytoplasm at pro/metaphase like the Miranda protein does, and the different routes taken by PON and Miranda are reflected in their differential requirement for myosin motors. In contrast to Miranda, PON localization depends on Myosin II but not on Myosin VI. The distinct localization modes of Miranda and PON might reflect their association with different cargoes and their intracellular localization. Miranda is required for the localization of transcriptional and translational regulators, such as Prospero and Brat, which presumably act in the cytoplasm and the nucleus, whereas PON localizes Numb, a regulator of Notch signaling that is primarily localized to the membrane or to cortical actin. Miranda also interacts with Numb in vitro (Shen et al., 1998); however, it is unclear whether this interaction plays a physiological role in the asymmetric localization of Numb (Ikeshima-Kataoka et al., 1997; Shen et al., 1997). The interaction of PON and Miranda at the basal cortex, however, appears to be similar, as suggested by FRAP analysis using PON- GFP and Miranda-GFP. Both proteins associate dynamically with the cortex, as indicated by their short half-time of recovery after photo-bleaching, but are retained within the limits of the basal cortical crescent (Fig. 4). Along these lines, FRAP analysis with GFP-PON, a fusion protein with the localization domain of PON, in sensory organ precursors of Drosophila pupae, suggested that there might be a constant exchange between cortical and cytoplasmic PON (Mayer et al., 2005). These data provide evidence for the presence of a common anchor protein that retains both PON and Miranda at the basal cortex. Taken together, we have identified distinguishable features between the localization machinery and the route for adaptor complexes in NBs. Further analyses studying the localization of cargo proteins, such as Prospero or Numb, are needed to establish whether PON and Miranda form two independently localized protein complexes. Myosin II and Myosin VI interact in one pathway to shuttle Miranda between cortex and cytoplasm Previous studies reported that Miranda forms an apical crescent at interphase or at prophase, and demonstrated a physical interaction of Miranda protein with Inscuteable, a component of the apical complex (Shen et al., 1998). It was therefore speculated that, after forming a complex with Prospero and Staufen at the apical cortex, Miranda receives a signal, presumably by interacting with Inscuteable, that triggers the Miranda complex to move towards the basal cortex. Here, we demonstrate that the apical crescent exists during interphase but rapidly disappears at early prophase. This suggests that Miranda receives the signal from the apical complex either during interphase or at early prophase. Recent data show that Myosin II and Lgl phosphorylated by apkc in the apical complex are required to exclude Miranda from the apical cortex. As previously shown, Myosin II is uniformly localized at the cortex during interphase (Barros et al., 2003) when Miranda is concentrated at the apical cortex (Fig. 1B). As active Myosin II has been suggested to exclude Miranda from the apical cortex, we propose that Myosin II is still inactive during interphase and does not exclude Miranda (Barros et al., 2003; Petritsch et al., 2003). Previous data Fig. 6. A model for Miranda localization by Myosin II and Myosin VI. (A) Late interphase. We propose the model that Myosin II forms an inactive crescent during late interphase (individual green ovals) because apkc is absent and cannot phosphorylate and inactivate Lgl (not shown). Myosin II binds to Miranda (red) in an apical crescent. PON is still cytoplasmic during interphase (yellow area). (B) Prophase and pro/metaphase. Very early at prophase, apkc binds to the apical crescent (purple crescent) and activates Myosin II to form microfilaments (connected green ovals) by phosphorylation of Lgl (not shown). Hence, Miranda is excluded from the apical cortex and mobilized to diffuse rapidly throughout the entire cytoplasm filling the nucleus around the time of nuclear envelope breakdown at pro/metaphase (red area). PON is recruited to the cortex (yellow circle) at prophase. (C) Metaphase. Myosin VI (blue) in the basal half of the cell binds to Miranda to either anchor it or to deliver Miranda by short-range transport to a cortical anchor at the basal crescent. PON is pushed along the cortex by Myosin II activity to form a basal crescent.
11 Dynamics of Miranda localization 1413 show that Myosin II interacts with Miranda in a protein complex in embryonic extracts (Petritsch et al., 2003). Because Myosin II and Miranda localization only overlaps during interphase, they might actually form a complex at the apical crescent during interphase. Indeed, apkc was rarely found in an apical crescent during interphase (Fig. 6A, data not shown), but it is recruited to an apical crescent at the transition of interphase to prophase (Fig. 1A, Fig. 6B). apkc in the apical complex phosphorylates Lgl, which allows for the activation of Myosin II at prophase (Betschinger et al., 2005; Barros et al., 2003). As suggested by our live imaging experiments (Fig. 6B), active Myosin II rapidly excludes Miranda from the cortex at early prophase. Miranda diffuses rapidly throughout the cytoplasm and, in line with earlier data (Petritsch et al., 2003), our live imaging analysis documented that Myosin VI is essential for the integration of cytoplasmically localized Miranda into the basal crescent at pro/metaphase (Fig. 5C, Fig. 6C). It is conceivable that Myosin VI binds to Miranda at juxta-cortical regions in the basal half of NBs and transports it over a short range to the basal cortex. Intriguingly, Myosin VI is capable of functioning not only as a processive motor but also as an anchor in vitro (Sweeney and Houdusse, 2007). Thus, alternatively, Myosin VI could retain the diffusing Miranda complex in the basal half of the metaphase NB, and thereby could facilitate delivery of Miranda to an as yet unknown cortical anchor (Mayer et al., 2005). As determination of the exact physiological function of Myosin VI in an individual cell type, like the NB, has not yet been attempted, we cannot currently distinguish between these possibilities. To address these questions, it will be necessary to study the interaction of Myosin VI and Miranda by mapping the interaction domains and measuring the fluorescent resonance energy transfer (FRET) between the two molecules. The distinct phenotype, mode of action, and subcellular localization of Myosin II and Myosin VI suggested that they act at distinct steps either in a single pathway or in two parallel pathways in the process of Miranda localization. Here, we have shown for the first time that Myosin II and Myosin VI act at consecutive steps in a single pathway to localize Miranda basally. Although many asymmetrically localized cell fate determinants, such as Numb, Prospero and Staufen, have mammalian homologs, mammalian proteins resembling Miranda or PON have yet to be found in neural stem cells of the mammalian brain. However, the high degree of conservation and the presence of asymmetric stem cell division suggest that functional homologs might exist, and that they are probably localized by similar mechanisms. Materials and Methods Fly strains For live imaging, full-length Miranda-GFP was expressed with the UAS/GAL4 system (Brand and Perrimon, 1993) using scabrous-gal4 (Nakao and Campos- Ortega, 1996), neuralized-gal4 (Bellaiche et al., 2001) or V32-Gal4 (Petritsch et al., 2003). We combined UAS-Miranda-GFP with red fluorescent histoneh2avdmrfp (Schuh et al., 2007) or with UAS-Lgl 3A (Betschinger et al., 2003). Live imaging for PON was performed with UAS-PON-GFP and sca-gal4 (Roegiers et al., 2001). FRAP experiments of free egfp used UAS-eGFP (Bloomington Stock Center). Generation of UAS-Miranda-GFP To generate a fusion of GFP and the N terminus of Miranda, a fragment encoding egfp, generated by PCR using egfp-vector (Clontech) as template, was fused to full-length Miranda, amplified by PCR from Miranda-bluescript-SK (Petritsch et al., 2003) with a small linker region, and cloned into the puast vector. Primer sequences were: egfp forward, 5 -ATAGAATTCGTAACGATGGTGAGCAAG-3 ; egfp reverse, 5 -TTACTCGAGGTCGACCTTGTACAG-3 ; Mira-full forward, 5 -ATC- TCGAGTCTTTCTCCAAG-3 ; and Mira-full reverse: 5 -ATGGTACC CTA GA - TGTTGCGTGC-3. Several independent transgenic lines were generated as described (Petritsch et al., 2003). Live imaging, FRAP and reduction of myosin activity Live embryos were dechorionated, immobilized on a cover slip (Lu et al., 1999), covered with Halocarbon 95 oil (Halocarbon Products) and visualized by confocal microscopy (Leica SP TCS_SP2; Objective, HCL PL APO lbd.bl Oil; numerical aperture, 1.4; room temperature C) using 6.5-second time intervals. NBs were identified as described previously (Kaltschmidt et al., 2000). To knock down myosin activity, embryos were injected with 17 mg/ml Rho-Kinase inhibitor Y (Tocris Biosciences) (Barros et al., 2003) and myosin VI dsrna (Petritsch et al., 2003), respectively. All photobleaching experiments were done by point bleaching of 1 second with maximum laser intensity using the advanced time-lapse tool. The recovery period was measured at lower laser intensity in time intervals of 3.25 seconds. For calculating the half-time of recovery, images were imported into image J and subtracted for background. The resulting curves were fitted to a single exponential function y=a(1-e-kt) with Origin 5.0 (Originlab), from which the FRAP half-time t 1/2 =ln(2)/k was calculated. Images were imported into Adobe Photoshop, assembled in Adobe Imageready and converted to QuickTime movies. Immunohistochemistry, drug treatment and in situ hybridization Antibody staining was as described (Patel, 1994), with the following modifications. Embryos were fixed for 4 minutes in a 1:1 mixture of 37% formaldehyde and heptane to preserve the cytoskeleton. Primary antibodies used were: rabbit anti-miranda 1:200 (Shen et al., 1997), mouse anti-miranda 1:20 (Ohshiro et al., 2000), rabbit anti-cyclin A 1:400 (a kind gift from P. O Farrell, University of California, San Francisco, CA), rabbit anti-pkcζ 1:200 (Santa Cruz Biotechnology), rabbit anti-α-tubulin 1:500 (Sigma Aldrich), rat anti-α-tubulin 1:10 (Accurate), rabbit anti-pon 1:1000 (Lu et al., 1998), and rabbit anti-γ-tubulin 1:1000 (Sigma Aldrich). Secondary antibodies directly coupled to Alexa (Molecular Probes) and Cy3 (Jackson) were used at 1:400 and 1:200, respectively. DNA was stained with TOTO-3 (1:1000; Molecular Probes). To inhibit proteasome activity, embryos were treated with 50 μm MG132 (Sigma) or DMSO as a vehicle control in a 1:1 mixture of Schneider s medium and heptane for 15 or 30 minutes. Whole mount in situ hybridization was performed as described (Tautz and Pfeifle, 1989). Following hybridization, embryos were incubated with mouse anti-dig (1:2000, Roche) and rabbit anti-miranda (1:100) antibodies, and secondary antibodies coupled to Alexa 488 and Cy3. Immunoblotting Miranda-GFP/scabrous-Gal4, Miranda-GFP and scabrous-gal4 embryos, respectively, were homogenized in extraction buffer [25 mm Hepes (ph 7), 50 mm KCl, 150 mm NaCl, 1 mm MgCl 2, 250 mm sucrose, 1 mm DTT, 1% Triton X-100 and protease inhibitor cocktail (Roche)] and lysates were subjected to western blotting according to standard procedures. Equal amounts of protein were loaded in each lane, and blots were probed with anti-miranda, anti-gfp and anti-α-tubulin antibodies. We thank M. Oft, F. Gao and F. Roegiers for critical reading of the manuscript; C. Lehner, S. Heidmann, F. Matsuzaki, F. Roegiers, Y. N. Jan, F. Schweisguth, B. Lu, S. Younger and the Bloomington Stock Center for fly strains; and M. McCleland, P. O Farrell and the Developmental Studies Hybridoma Bank for antibodies. We are grateful to L. Kockel, Y. Lin and K. Brückner for embryos and fly food; H. Leonhardt and the Leonhardt lab for suggestions with FRAP analysis; and R. Heptner for technical assistance. This work was supported by grants to C.P. from the Deutsche Forschungsgemeinschaft (SFB 413 and SFB 646). References Barros, C. S., Phelps, C. B. and Brand, A. H. (2003). Drosophila nonmuscle myosin II promotes the asymmetric segregation of cell fate determinants by cortical exclusion rather than active transport. Dev. Cell 5, Bellaiche, Y., Gho, M., Kaltschmidt, J. A., Brand, A. H. and Schweisguth, F. (2001). Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat. Cell Biol. 3, Betschinger, J. and Knoblich, J. A. (2004). Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674-R685. Betschinger, J., Mechtler, K. and Knoblich, J. A. (2003). The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422, Betschinger, J., Eisenhaber, F. and Knoblich, J. A. (2005). Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr. Biol. 15, Brand, A. H. and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, Bresnick, A. R. (1999). Molecular mechanisms of nonmuscle myosin-ii regulation. Curr. Opin. Cell Biol. 11,
Cosa sono le cellule staminali?
 Cosa sono le cellule staminali? Cellule Staminali Embrionali: totipotenti o pluripotenti Provengono dalla inner cell mass di embrioni di 4-5 giorni (blastocisti) prodotti in vitro Adulte: multipotenti
Cosa sono le cellule staminali? Cellule Staminali Embrionali: totipotenti o pluripotenti Provengono dalla inner cell mass di embrioni di 4-5 giorni (blastocisti) prodotti in vitro Adulte: multipotenti
Section 10. Junaid Malek, M.D.
 Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit
 Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
CHAPTER 16 THE CYTOSKELETON
 CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
Chapter 3. DNA Replication & The Cell Cycle
 Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
Chapter 3 DNA Replication & The Cell Cycle DNA Replication and the Cell Cycle Before cells divide, they must duplicate their DNA // the genetic material DNA is organized into strands called chromosomes
Human Anatomy & Physiology I Dr. Sullivan Unit IV Cellular Function Chapter 4, Chapter 27 (meiosis only)
 Human Anatomy & Physiology I Dr. Sullivan Unit IV Cellular Function Chapter 4, Chapter 27 (meiosis only) I. Protein Synthesis: creation of new proteins a. Much of the cellular machinery is devoted to synthesizing
Human Anatomy & Physiology I Dr. Sullivan Unit IV Cellular Function Chapter 4, Chapter 27 (meiosis only) I. Protein Synthesis: creation of new proteins a. Much of the cellular machinery is devoted to synthesizing
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15
 Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
Supplemental Fig. 1. Mcr alleles show defects in tracheal tube size and luminal protein accumulation. (A-F) Confocal projections of living stage 15 embryos expressing GFP and Verm-RFP in tracheal cells
Quantitative Analysis of Protein Dynamics during Asymmetric Cell Division
 Current Biology, Vol. 15, 1847 1854, October 25, 2005, 2005 Elsevier Ltd All rights reserved. DOI 10.1016/j.cub.2005.08.067 Quantitative Analysis of Protein Dynamics during Asymmetric Cell Division Bernd
Current Biology, Vol. 15, 1847 1854, October 25, 2005, 2005 Elsevier Ltd All rights reserved. DOI 10.1016/j.cub.2005.08.067 Quantitative Analysis of Protein Dynamics during Asymmetric Cell Division Bernd
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
Supplemental material Wang et al., http://www.jcb.org/cgi/content/full/jcb.201405026/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Generation and characterization of unc-40 alleles. (A and
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Figure S2. Verification of ihog and boi
 Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
Figure S1. Verification of ihog Mutation by Protein Immunoblotting Extracts from S2R+ cells, embryos, and adults were analyzed by immunoprecipitation and immunoblotting with anti-ihog antibody. The Ihog
Fig. S1. Endocytosis of extracellular cargo does not depend on dia. (A) To visualize endocytosis, fluorescently labelled wheat germ agglutinin
 Fig. S1. Endocytosis of extracellular cargo does not depend on dia. (A) To visualize endocytosis, fluorescently labelled wheat germ agglutinin (WGA-Alexa555) was injected into the extracellular perivitteline
Fig. S1. Endocytosis of extracellular cargo does not depend on dia. (A) To visualize endocytosis, fluorescently labelled wheat germ agglutinin (WGA-Alexa555) was injected into the extracellular perivitteline
Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product.
 Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
Supplementary Information Supplementary Figure S1. Immunodetection of full-length XA21 and the XA21 C-terminal cleavage product. Total protein extracted from Kitaake wild type and rice plants carrying
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
indicated numbers of pups at day of life (DOL) 10, or embryonic day (ED) B. Male mice of
 SUPPLEMENTRY FIGURE LEGENDS Figure S1. USP44 loss leads to chromosome missegregation.. Genotypes obtained from the indicated numbers of pups at day of life (DOL) 10, or embryonic day (ED) 13.5.. Male mice
SUPPLEMENTRY FIGURE LEGENDS Figure S1. USP44 loss leads to chromosome missegregation.. Genotypes obtained from the indicated numbers of pups at day of life (DOL) 10, or embryonic day (ED) 13.5.. Male mice
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
1. I can describe the stages of the cell cycle.
 Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
(phosphatase tensin) domain is shown in dark gray, the FH1 domain in black, and the
 Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table.
 Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Cell Cycle. Trends in Cell Biology
 Cell Cycle Trends in Cell Biology Cyclic proteolysis Two distinct enzyme complexes proteolysis of cyclin-cdk complexes SCF (Skp1-Cul1-F box) Ubiquitylation and destruction of G1/S-cyclins and CKI proteins
Cell Cycle Trends in Cell Biology Cyclic proteolysis Two distinct enzyme complexes proteolysis of cyclin-cdk complexes SCF (Skp1-Cul1-F box) Ubiquitylation and destruction of G1/S-cyclins and CKI proteins
C. Incorrect! Second Law: Law of Independent Assortment - Genes for different traits sort independently of one another in the formation of gametes.
 OAT Biology - Problem Drill 20: Chromosomes and Genetic Technology Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick
OAT Biology - Problem Drill 20: Chromosomes and Genetic Technology Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick
Supporting information
 Supporting information Construction of strains and plasmids To create ptc67, a PCR product obtained with primers cc2570-162f (gcatgggcaagcttgaggacggcgtcatgt) and cc2570+512f (gaggccgtggtaccatagaggcgggcg),
Supporting information Construction of strains and plasmids To create ptc67, a PCR product obtained with primers cc2570-162f (gcatgggcaagcttgaggacggcgtcatgt) and cc2570+512f (gaggccgtggtaccatagaggcgggcg),
Lecture 20: Drosophila melanogaster
 Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
1. I can describe the stages of the cell cycle.
 Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Unit 5 Study Guide Cell Cycle pg. 1 1. I can describe the stages of the cell cycle. Interphase = period in between division G1 = growth phase S = DNA replication G2 = Preparation for division (extra copies
Practice Problems 5. Location of LSA-GFP fluorescence
 Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
CELLULAR PROCESSES; REPRODUCTION. Unit 5
 CELLULAR PROCESSES; REPRODUCTION Unit 5 Cell Cycle Chromosomes and their make up Crossover Cytokines Diploid (haploid diploid and karyotypes) Mitosis Meiosis What is Cancer? Somatic Cells THE CELL CYCLE
CELLULAR PROCESSES; REPRODUCTION Unit 5 Cell Cycle Chromosomes and their make up Crossover Cytokines Diploid (haploid diploid and karyotypes) Mitosis Meiosis What is Cancer? Somatic Cells THE CELL CYCLE
(a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for
 Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
A Genetic Screen to Identify Mammalian Chromatin Modifiers In Vivo.
 Gus Frangou, Stephanie Palmer & Mark Groudine Fred Hutchinson Cancer Research Center, Seattle- USA A Genetic Screen to Identify Mammalian Chromatin Modifiers In Vivo. During mammalian development and differentiation
Gus Frangou, Stephanie Palmer & Mark Groudine Fred Hutchinson Cancer Research Center, Seattle- USA A Genetic Screen to Identify Mammalian Chromatin Modifiers In Vivo. During mammalian development and differentiation
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Animal Development Regulation of gene expression
 Animal Development Regulation of gene expression Prof. Ilan Davis, Department of Biochemistry. Wellcome Trust Research Senior Fellow Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com
Animal Development Regulation of gene expression Prof. Ilan Davis, Department of Biochemistry. Wellcome Trust Research Senior Fellow Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com
Supplemental Material
 Supplemental Material 1 Figure S1. Phylogenetic analysis of Cep72 and Lrrc36, comparative localization of Cep72 and Lrrc36 and Cep72 antibody characterization (A) Phylogenetic alignment of Cep72 and Lrrc36
Supplemental Material 1 Figure S1. Phylogenetic analysis of Cep72 and Lrrc36, comparative localization of Cep72 and Lrrc36 and Cep72 antibody characterization (A) Phylogenetic alignment of Cep72 and Lrrc36
T he process of unequal segregation of determinants first
 Frizzled regulates localization of cellfate determinants and mitotic spindle rotation during asymmetric cell division Yohanns Bellaïche*, Michel Gho*, Julia A. Kaltschmidt, Andrea H. Brand and François
Frizzled regulates localization of cellfate determinants and mitotic spindle rotation during asymmetric cell division Yohanns Bellaïche*, Michel Gho*, Julia A. Kaltschmidt, Andrea H. Brand and François
To investigate the heredity of the WFP gene, we selected plants that were homozygous
 Supplementary information Supplementary Note ST-12 WFP allele is semi-dominant To investigate the heredity of the WFP gene, we selected plants that were homozygous for chromosome 1 of Nipponbare and heterozygous
Supplementary information Supplementary Note ST-12 WFP allele is semi-dominant To investigate the heredity of the WFP gene, we selected plants that were homozygous for chromosome 1 of Nipponbare and heterozygous
Cell cycle oscillations
 Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
Figure S1. Figure S2. Figure S3 HB Anti-FSP27 (COOH-terminal peptide) Ab. Anti-GST-FSP27(45-127) Ab.
 / 36B4 mrna ratio Figure S1 * 2. 1.6 1.2.8 *.4 control TNFα BRL49653 Figure S2 Su bw AT p iw Anti- (COOH-terminal peptide) Ab Blot : Anti-GST-(45-127) Ab β-actin Figure S3 HB2 HW AT BA T Figure S4 A TAG
/ 36B4 mrna ratio Figure S1 * 2. 1.6 1.2.8 *.4 control TNFα BRL49653 Figure S2 Su bw AT p iw Anti- (COOH-terminal peptide) Ab Blot : Anti-GST-(45-127) Ab β-actin Figure S3 HB2 HW AT BA T Figure S4 A TAG
Trasposable elements: Uses of P elements Problem set B at the end
 Trasposable elements: Uses of P elements Problem set B at the end P-elements have revolutionized the way Drosophila geneticists conduct their research. Here, we will discuss just a few of the approaches
Trasposable elements: Uses of P elements Problem set B at the end P-elements have revolutionized the way Drosophila geneticists conduct their research. Here, we will discuss just a few of the approaches
Supplementary Figure S1. N-terminal fragments of LRRK1 bind to Grb2.
 Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Myc- HA-Grb2 Mr(K) 105 IP HA 75 25 105 1-1163 1-595 - + - + - + 1164-1989 Blot Myc HA total lysate 75 25 Myc HA Supplementary Figure S1. N-terminal fragments of bind to Grb2. COS7 cells were cotransfected
Nature Biotechnology: doi: /nbt.4166
 Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
Supplementary Figure 1 Validation of correct targeting at targeted locus. (a) by immunofluorescence staining of 2C-HR-CRISPR microinjected embryos cultured to the blastocyst stage. Embryos were stained
Section C: The Control of Gene Expression
 Section C: The Control of Gene Expression 1. Each cell of a multicellular eukaryote expresses only a small fraction of its genes 2. The control of gene expression can occur at any step in the pathway from
Section C: The Control of Gene Expression 1. Each cell of a multicellular eukaryote expresses only a small fraction of its genes 2. The control of gene expression can occur at any step in the pathway from
Cell Division. embryo: an early stage of development in organisms
 Over the past several years, a debate has been brewing over the use of stem cells. Stem cells can be used to treat certain diseases and conditions such as spinal cord injuries, diabetes, arthritis, and
Over the past several years, a debate has been brewing over the use of stem cells. Stem cells can be used to treat certain diseases and conditions such as spinal cord injuries, diabetes, arthritis, and
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Disengaging the Smc3/kleisin interface releases cohesin from Drosophila chromosomes during interphase and mitosis
 Manuscript EMBO-2012-83126 Disengaging the Smc3/kleisin interface releases cohesin from Drosophila chromosomes during interphase and mitosis Christian S. Eichinger, Alexander Kurze, Raquel A. Oliveira,
Manuscript EMBO-2012-83126 Disengaging the Smc3/kleisin interface releases cohesin from Drosophila chromosomes during interphase and mitosis Christian S. Eichinger, Alexander Kurze, Raquel A. Oliveira,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10810 Supplementary Fig. 1: Mutation of the loqs gene leads to shortened lifespan and adult-onset brain degeneration. a. Northern blot of control and loqs f00791 mutant flies. loqs f00791
doi:10.1038/nature10810 Supplementary Fig. 1: Mutation of the loqs gene leads to shortened lifespan and adult-onset brain degeneration. a. Northern blot of control and loqs f00791 mutant flies. loqs f00791
Supplemental Information. Plk1/Polo Phosphorylates Sas-4 at the Onset. of Mitosis for an Efficient Recruitment
 Cell Reports, Volume 25 Supplemental Information Plk1/Polo Phosphorylates Sas-4 at the Onset of Mitosis for an Efficient Recruitment of Pericentriolar Material to Centrosomes Anand Ramani, Aruljothi Mariappan,
Cell Reports, Volume 25 Supplemental Information Plk1/Polo Phosphorylates Sas-4 at the Onset of Mitosis for an Efficient Recruitment of Pericentriolar Material to Centrosomes Anand Ramani, Aruljothi Mariappan,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
SUPPLEMENTARY INFORMATION Legends for Supplementary Tables. Supplementary Table 1. An excel file containing primary screen data. Worksheet 1, Normalized quantification data from a duplicated screen: valid
To assess the localization of Citrine fusion proteins, we performed antibody staining to
 Trinh et al 1 SUPPLEMENTAL MATERIAL FlipTraps recapitulate endogenous protein localization To assess the localization of Citrine fusion proteins, we performed antibody staining to compare the expression
Trinh et al 1 SUPPLEMENTAL MATERIAL FlipTraps recapitulate endogenous protein localization To assess the localization of Citrine fusion proteins, we performed antibody staining to compare the expression
The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts
 The NuMA-related protein binds and regulates spindle orientation in Drosophila neuroblasts Karsten H. Siller 1, Clemens Cabernard 1 and Chris Q. Doe 1,2 Asymmetric cell division generates cell diversity
The NuMA-related protein binds and regulates spindle orientation in Drosophila neuroblasts Karsten H. Siller 1, Clemens Cabernard 1 and Chris Q. Doe 1,2 Asymmetric cell division generates cell diversity
Identification of Miranda Associated Proteins and RNA in Drosophila melanogaster Neuroblasts
 Dissertation zur Erlangung des Doktorgrades der Fakultät für Chemie und Pharmazie der Ludwig-Maximilians-Universität München Identification of Miranda Associated Proteins and RNA in Drosophila melanogaster
Dissertation zur Erlangung des Doktorgrades der Fakultät für Chemie und Pharmazie der Ludwig-Maximilians-Universität München Identification of Miranda Associated Proteins and RNA in Drosophila melanogaster
environment (diffusion, etc.). High SA:V ratio is favorable. Ex. 6:1 is better than 6:5
 Page 21 AP Biology: 2013 Exam Review CONCEPT 4 THE CELL CYCLE AND HEREDITY 1. Cell cycle a. Reason for division- as cells increase in volume, the surface area decreases and demand for material resources
Page 21 AP Biology: 2013 Exam Review CONCEPT 4 THE CELL CYCLE AND HEREDITY 1. Cell cycle a. Reason for division- as cells increase in volume, the surface area decreases and demand for material resources
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling
 Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling Go-Woon Kim, Jong-Hoon Won, Ok-Kyung Lee, Sang-Soo Lee, Jeong-Hoon Han, Orkhon Tsogtbaatar, Sujin Nam, Yeon Kim, and Kyung-Ok Cho* Supplementary
Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling Go-Woon Kim, Jong-Hoon Won, Ok-Kyung Lee, Sang-Soo Lee, Jeong-Hoon Han, Orkhon Tsogtbaatar, Sujin Nam, Yeon Kim, and Kyung-Ok Cho* Supplementary
Supplemental Figure 1 Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical
 Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
Supplemental Figure Human REEP family of proteins can be divided into two distinct subfamilies. Residues (single letter amino acid code) identical in all six REEPs are highlighted in green. Additional
Characterization of a novel gene involved in border cell migration
 Characterization of a novel gene involved in border cell migration Wei-Hao Li, He-Yen Chou and Li-Mei Pai Graduate Institute of Basic Medical Science Chang-Gung University, Tao-Yuan, Taiwan, R.O.C. Abstract
Characterization of a novel gene involved in border cell migration Wei-Hao Li, He-Yen Chou and Li-Mei Pai Graduate Institute of Basic Medical Science Chang-Gung University, Tao-Yuan, Taiwan, R.O.C. Abstract
Learning Objectives. Define RNA interference. Define basic terminology. Describe molecular mechanism. Define VSP and relevance
 Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
7.06 Problem Set Four, 2006
 7.06 Problem Set Four, 2006 1. Explain the molecular mechanism behind each of the following events that occur during the cell cycle, making sure to discuss specific proteins that are involved in making
7.06 Problem Set Four, 2006 1. Explain the molecular mechanism behind each of the following events that occur during the cell cycle, making sure to discuss specific proteins that are involved in making
SUPPLEMENTARY INFORMATION
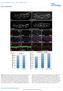 Normalized dorsal trunk length (µm) Normalized dorsal trunk length (TC1-5) (µm) DOI: 1.138/ncb2467 a b c d btl>src64 Src42, btl>src64 e Src64 f g h DT Src64 DT Midgut Src64 Midgut i Src64 Atpα j k l DT
Normalized dorsal trunk length (µm) Normalized dorsal trunk length (TC1-5) (µm) DOI: 1.138/ncb2467 a b c d btl>src64 Src42, btl>src64 e Src64 f g h DT Src64 DT Midgut Src64 Midgut i Src64 Atpα j k l DT
PHT1;2-CFP YFP-PHF + PHT1;2-CFP YFP-PHF
 YFP-PHF1 CFP-PHT1;2 PHT1;2-CFP YFP-PHF + PHT1;2-CFP YFP-PHF + CFP-PHT1;2 Negative control!-gfp Supplemental Figure 1: PHT1;2 accumulation is PHF1 dependent. Immunoblot analysis on total protein extract
YFP-PHF1 CFP-PHT1;2 PHT1;2-CFP YFP-PHF + PHT1;2-CFP YFP-PHF + CFP-PHT1;2 Negative control!-gfp Supplemental Figure 1: PHT1;2 accumulation is PHF1 dependent. Immunoblot analysis on total protein extract
7.17: Writing Up Results and Creating Illustrations
 7.17: Writing Up Results and Creating Illustrations A Results Exercise: Kansas and Pancakes Write a 5-sentence paragraph describing the results illustrated in this figure: - Describe the figure: highlights?
7.17: Writing Up Results and Creating Illustrations A Results Exercise: Kansas and Pancakes Write a 5-sentence paragraph describing the results illustrated in this figure: - Describe the figure: highlights?
PRINCIPLES O F NUCLEAR STRUCTUR E AND FUNCTIO N. Peter R. Cook
 PRINCIPLES O F NUCLEAR STRUCTUR E AND FUNCTIO N Peter R. Cook Preface xii i Acknowledgments xv 1. SOME PRINCIPLES 1 Overview of the Cell Nucleus 1 Box 1-1. Discovery of Cells, Nuclei, and DNA 2 A Sense
PRINCIPLES O F NUCLEAR STRUCTUR E AND FUNCTIO N Peter R. Cook Preface xii i Acknowledgments xv 1. SOME PRINCIPLES 1 Overview of the Cell Nucleus 1 Box 1-1. Discovery of Cells, Nuclei, and DNA 2 A Sense
Description: Nuclear morphology and dynamics in nontargeting sirna transfected cells. HeLa Kyoto
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Tables Title of file for HTML: Supplementary Movie 1 Description: Nuclear morphology and dynamics
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Tables Title of file for HTML: Supplementary Movie 1 Description: Nuclear morphology and dynamics
The N terminus of the Drosophila Numb protein directs membrane association and actin-dependent asymmetric localization
 Proc. Natl. Acad. Sci. USA Vol. 94, pp. 13005 13010, November 1997 Developmental Biology The N terminus of the Drosophila Numb protein directs membrane association and actin-dependent asymmetric localization
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 13005 13010, November 1997 Developmental Biology The N terminus of the Drosophila Numb protein directs membrane association and actin-dependent asymmetric localization
supplementary information
 DOI: 10.1038/ncb1919 Mori et al. Supplementary Figure 1a-b a Characterization of the mdrg cells Anti-NF150 DIC (89%: N=154) 100µm Anti-S100 DIC 100µm b (9.1%: N=144) Characterization of the cortical neurons
DOI: 10.1038/ncb1919 Mori et al. Supplementary Figure 1a-b a Characterization of the mdrg cells Anti-NF150 DIC (89%: N=154) 100µm Anti-S100 DIC 100µm b (9.1%: N=144) Characterization of the cortical neurons
Supporting Information
 Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
7.06 Problem Set #3, Spring 2005
 7.06 Problem Set #3, Spring 2005 1. The Drosophila compound eye is composed of about 800 units called ommatidia. Each ommatidium contains eight photoreceptor neurons (R1 through R8), which develop in a
7.06 Problem Set #3, Spring 2005 1. The Drosophila compound eye is composed of about 800 units called ommatidia. Each ommatidium contains eight photoreceptor neurons (R1 through R8), which develop in a
Supplementary Figure 1. Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings.
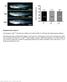 Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
transcription and the promoter occupancy of Smad proteins. (A) HepG2 cells were co-transfected with the wwp-luc reporter, and FLAG-tagged FHL1,
 Supplementary Data Supplementary Figure Legends Supplementary Figure 1 FHL-mediated TGFβ-responsive reporter transcription and the promoter occupancy of Smad proteins. (A) HepG2 cells were co-transfected
Supplementary Data Supplementary Figure Legends Supplementary Figure 1 FHL-mediated TGFβ-responsive reporter transcription and the promoter occupancy of Smad proteins. (A) HepG2 cells were co-transfected
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Paul et al.,
 Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
Biology 4361 Developmental Biology Differential Gene Expression September 28, 2006 Chromatin Structure ~140 bp ~60 bp Transcriptional Regulation: 1. Packing prevents access CH 3 2. Acetylation ( C O )
Vocab Word 1: Interphase
 Vocab Word 1: Interphase Interphase is the phase of the cell cycle in which a typical cell spends most of its life. During this phase, the cell copies its DNA in preparation for mitosis. Interphase is
Vocab Word 1: Interphase Interphase is the phase of the cell cycle in which a typical cell spends most of its life. During this phase, the cell copies its DNA in preparation for mitosis. Interphase is
New Tool for Late Stage Visualization of Oskar Protein in Drosophila melanogaster. Oocytes. Kathleen Harnois. Supervised by Paul Macdonald
 New Tool for Late Stage Visualization of Oskar Protein in Drosophila melanogaster Oocytes Kathleen Harnois Supervised by Paul Macdonald Institute of Cellular and Molecular Biology, The University of Texas
New Tool for Late Stage Visualization of Oskar Protein in Drosophila melanogaster Oocytes Kathleen Harnois Supervised by Paul Macdonald Institute of Cellular and Molecular Biology, The University of Texas
Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. A. Fire et al. (1998) Nature Vol 391:
 Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans A. Fire et al. (1998) Nature Vol 391: 806-810 1 Outline 1. Introduction 2. Objective 3. Results 4. Mechanism 5.
Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans A. Fire et al. (1998) Nature Vol 391: 806-810 1 Outline 1. Introduction 2. Objective 3. Results 4. Mechanism 5.
DNA STRUCTURE. Nucleotides: Nitrogenous Bases (Carry the Genetic Code) Expectation Sheet: DNA & Cell Cycle. I can statements: Basic Information:
 Expectation Sheet: DNA & Cell Cycle NAME: Test is 11/8/17 I can statements: I can discuss how DNA is found in all organisms and that the structure is common to all living things. I can diagram and label
Expectation Sheet: DNA & Cell Cycle NAME: Test is 11/8/17 I can statements: I can discuss how DNA is found in all organisms and that the structure is common to all living things. I can diagram and label
Two classes of silencing RNAs move between Caenorhabditis elegans tissues.
 Two classes of silencing RNAs move between Caenorhabditis elegans tissues. Antony M Jose, Giancarlo A Garcia, and Craig P Hunter. Supplementary Figures, Figure Legends, and Tables. Supplementary Figure
Two classes of silencing RNAs move between Caenorhabditis elegans tissues. Antony M Jose, Giancarlo A Garcia, and Craig P Hunter. Supplementary Figures, Figure Legends, and Tables. Supplementary Figure
Lecture 20: Drosophila embryogenesis
 Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
Differential Gene Expression
 Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
Biology 4361 Developmental Biology Differential Gene Expression June 19, 2008 Differential Gene Expression Overview Chromatin structure Gene anatomy RNA processing and protein production Initiating transcription:
NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function
 Manuscript EMBO-2013-85125 NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function Sachin Kotak, Coralie Busso, Pierre Gönczy Corresponding author: Pierre Gönczy, ISREC Review
Manuscript EMBO-2013-85125 NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function Sachin Kotak, Coralie Busso, Pierre Gönczy Corresponding author: Pierre Gönczy, ISREC Review
A functional analysis of inscuteable and its roles during Drosophila asymmetric cell divisions
 Journal of Cell Science 112, 1541-1551 (1999) Printed in Great Britain The Company of Biologists Limited 1999 JCS3885 1541 A functional analysis of inscuteable and its roles during Drosophila asymmetric
Journal of Cell Science 112, 1541-1551 (1999) Printed in Great Britain The Company of Biologists Limited 1999 JCS3885 1541 A functional analysis of inscuteable and its roles during Drosophila asymmetric
Supplemental Information
 Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Supplemental Information Itemized List Materials and Methods, Related to Supplemental Figures S5A-C and S6. Supplemental Figure S1, Related to Figures 1 and 2. Supplemental Figure S2, Related to Figure
Cell cycle. Chen Li. Department of cellular and genetic medicine
 Cell cycle Chen Li Department of cellular and genetic medicine 13 223 chenli2008@fudan.edu.cn Outline A. Historical background B. Phases of cell cycle C. DNA replication D. Telomere & telomerase E. DNA
Cell cycle Chen Li Department of cellular and genetic medicine 13 223 chenli2008@fudan.edu.cn Outline A. Historical background B. Phases of cell cycle C. DNA replication D. Telomere & telomerase E. DNA
 DOI: 10.1038/ncb3259 A Ismail et al. Supplementary Figure 1 B 60000 45000 SSC 30000 15000 Live cells 0 0 15000 30000 45000 60000 FSC- PARR 60000 45000 PARR Width 30000 FSC- 15000 Single cells 0 0 15000
DOI: 10.1038/ncb3259 A Ismail et al. Supplementary Figure 1 B 60000 45000 SSC 30000 15000 Live cells 0 0 15000 30000 45000 60000 FSC- PARR 60000 45000 PARR Width 30000 FSC- 15000 Single cells 0 0 15000
supplementary information
 DOI: 10.1038/ncb2156 Figure S1 Depletion of p114rhogef with different sirnas. Caco-2 (a) and HCE (b) cells were transfected with individual sirnas, pools of the two sirnas or the On Target (OnT) sirna
DOI: 10.1038/ncb2156 Figure S1 Depletion of p114rhogef with different sirnas. Caco-2 (a) and HCE (b) cells were transfected with individual sirnas, pools of the two sirnas or the On Target (OnT) sirna
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.
 Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
Fig. S1. Effect of p120-catenin overexpression on the interaction of SCUBE2 with E-cadherin. The expression plasmid encoding FLAG.SCUBE2, E-cadherin.Myc, or HA.p120-catenin was transfected in a combination
Enhancers mutations that make the original mutant phenotype more extreme. Suppressors mutations that make the original mutant phenotype less extreme
 Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full//59/ra7/dc1 Supplementary Materials for Dok-7 ctivates the Muscle Receptor Kinase MuSK and Shapes Synapse Formation kane Inoue, Kiyoko Setoguchi, Yosuke Matsubara,
www.sciencesignaling.org/cgi/content/full//59/ra7/dc1 Supplementary Materials for Dok-7 ctivates the Muscle Receptor Kinase MuSK and Shapes Synapse Formation kane Inoue, Kiyoko Setoguchi, Yosuke Matsubara,
Enzyme that uses RNA as a template to synthesize a complementary DNA
 Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Comparison of two or more protein or DNA sequence to ascertain similarities in sequences. If two genes have
Biology 105: Introduction to Genetics PRACTICE FINAL EXAM 2006 Part I: Definitions Homology: Comparison of two or more protein or DNA sequence to ascertain similarities in sequences. If two genes have
Figure S1 is related to Figure 1B, showing more details of outer segment of
 Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
Test Bank for Molecular Cell Biology 7th Edition by Lodish
 Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and
 Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
Supplementary Fig. S1. Building a training set of cardiac enhancers. (A-E) Empirical validation of candidate enhancers containing matches to Twi and Tin TFBS motifs and located in the flanking or intronic
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
These molecules make up the ladder of the DNA Bound by weak hydrogen bonds. 4 Different Types (2 specific matches) look at the
 B.A.T. Review DNA & Cell Cycle Test is 11/3/16 NAME: PERIOD DNA STRUCTURE VOCABULARY Mitosis Chromosomes Interphase G1,S,G2,M Prophase Metaphase Anaphase Telophase Cytokinesis Cleavage Furrow Spindle Fibers
B.A.T. Review DNA & Cell Cycle Test is 11/3/16 NAME: PERIOD DNA STRUCTURE VOCABULARY Mitosis Chromosomes Interphase G1,S,G2,M Prophase Metaphase Anaphase Telophase Cytokinesis Cleavage Furrow Spindle Fibers
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11070 Supplementary Figure 1 Purification of FLAG-tagged proteins. a, Purification of FLAG-RNF12 by FLAG-affinity from nuclear extracts of wild-type (WT) and two FLAG- RNF12 transgenic
doi:10.1038/nature11070 Supplementary Figure 1 Purification of FLAG-tagged proteins. a, Purification of FLAG-RNF12 by FLAG-affinity from nuclear extracts of wild-type (WT) and two FLAG- RNF12 transgenic
Chapter 14 Regulation of Transcription
 Chapter 14 Regulation of Transcription Cis-acting sequences Distance-independent cis-acting elements Dissecting regulatory elements Transcription factors Overview transcriptional regulation Transcription
Chapter 14 Regulation of Transcription Cis-acting sequences Distance-independent cis-acting elements Dissecting regulatory elements Transcription factors Overview transcriptional regulation Transcription
